US Pharm. 2020;45(7/8):36-40.
ABSTRACT: The accessible and informed pharmacist plays an increasingly important role in fostering infant health and nutrition. Parents need prompt, reliable, and continuous evidence-based information about the benefits of breastfeeding for both the mother and the child. Human milk is the optimal source of nutrition for infants, but in some cases mothers opt to formula feed, and there are some circumstances in which formula feeding may be preferable or necessary. Pharmacists can contribute to infants’ optimal growth and development by providing parents with information on breastfeeding, indications for formula feeding, and the introduction of complementary foods and vitamin and mineral supplementation.
The involvement of accessible and informed healthcare professionals such as community pharmacists is increasingly important for infant health and nutrition. Parents need prompt, reliable, and continuous evidence-based information concerning breastfeeding, supplementation, and nutrition so that they can make informed choices. The pharmacy is often the first port of call for new parents, and pharmacists are in a unique position to listen to their concerns and be equipped with the appropriate resources to best assist them.
Human Milk
Human milk is the optimal source of nutrition for infants. Pharmacists can play an important role in encouraging breastfeeding by supporting the natural process of infant feeding, removing barriers to success, and supplying additional resources when challenges arise. As the most accessible and trusted community healthcare providers, pharmacists can provide early access to support and resources, which is critical, especially when new mothers feel overwhelmed. Pharmacists can counsel pregnant and postpartum women on breastfeeding recommendations based on guidelines and resources from the American Academy of Pediatrics (AAP). The AAP recommends that infants be breastfed for 6 months, followed by a combination of breastfeeding plus complementary foods for at least 1 year, and beyond that as desired. These recommendations are strongly supported by multiple medical and professional organizations, including the American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the World Health Organization (WHO), and the Canadian Pediatric Society, on the basis of short- and long-term benefits for both mother and child. Additionally, the WHO recommends that breastfeeding be continued at least through the child’s second birthday.1,2
Human milk is much more complex than the sum of its nutritional components. Its components have antimicrobial and immunomodulatory properties and include factors that promote gastrointestinal (GI) development. In addition to its many nutrients, human milk contains living cells, growth factors, and immunoprotective substances. Many of these factors are resistant to digestive enzymes in the infant’s GI tract and are biologically active at mucosal surfaces. Compared with infant formula, human milk also appears to provide continued protection against acute illnesses, such as otitis media and pneumonia, even after breastfeeding is discontinued. Breastfeeding has been associated with long-term benefits by reducing risk for several chronic diseases, including type 1 diabetes mellitus, inflammatory bowel disease, and wheezing in young children. Additional protective effects against obesity, atopic asthma, eczema, food allergies, leukemia, and neurodevelopmental outcomes may exist but are not as well established in the literature.2-10
Despite these benefits, common reasons given by mothers for early cessation of breastfeeding include their milk supply and difficulty with breastfeeding technique. Moreover, some mothers may decide to formula-feed because of a lack of support, health issues, convenience, specialized need, or economic pressure to return to work early. However, state and federal laws have been enacted that require workplaces to better accommodate and support mothers transitioning back to the workplace, such as providing reasonable breaktimes and nonrestroom areas where they can pump breast milk. Importantly, many issues that arise could be solved or avoided if mothers were better supported by the community. In such cases, to optimize outcomes and resolve problems early, the pharmacist can step in as both patient advocate and liaison between patients and various community groups providing local mother-to-mother peer support (e.g., La Leche League) and local lactation consultants. Most problems with breastfeeding stem from difficulties relating to lack of appropriate information and the mother’s lack of confidence, especially in first-time mothers, and the pharmacist has a unique opportunity to provide support and guidance, given the right toolkit.1,11
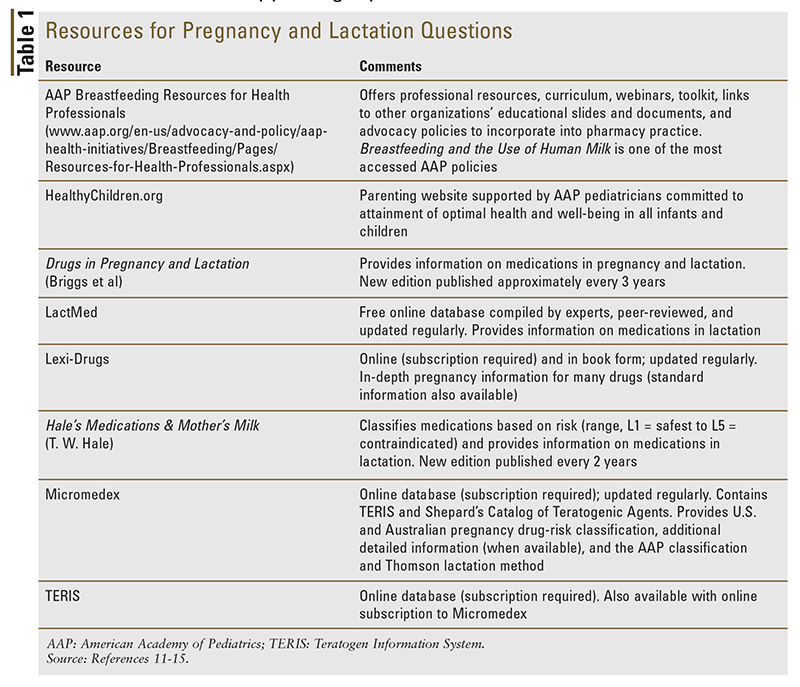
It is important to note that there are a few circumstances in which breastfeeding is inadvisable and needs to be discontinued or discouraged. These selected cases include the infant whose mother has untreated and active tuberculosis; the infant with a classic form of galactosemia; and—in developed countries—the infant whose mother has HIV. Also, although most medications are compatible with breastfeeding, certain medications may make it necessary to interrupt breastfeeding. In these situations, the pharmacist can closely collaborate with the mother’s primary care provider to weigh the risks versus benefits and identify potential safer agents to substitute so that the mother can breastfeed. To this end, the pharmacist may use a practical set of tools to review the available information and evaluate the risks of using or withholding a particular medication (TABLE 1).1,11-15
Infant Formula
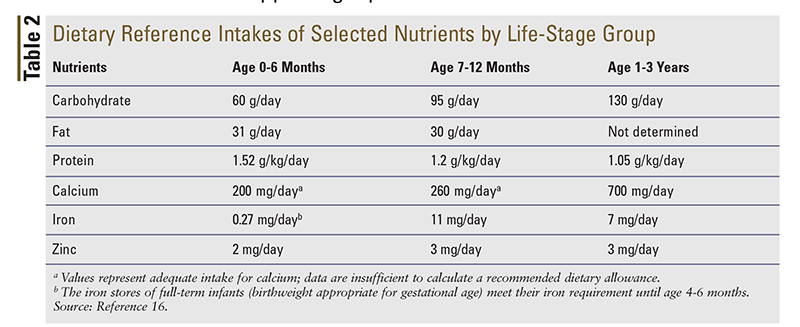
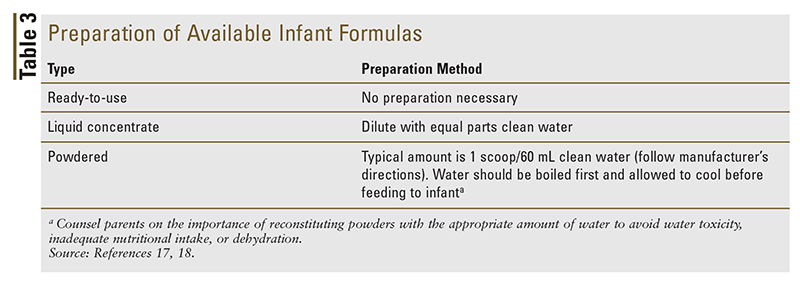
Although they typically may not receive an abundance of questions regarding initiation of infant formula, pharmacists can be a valuable and proactive resource for caregivers who are confused and intimidated by the vast selection of available infant formulas. By understanding the differences in formula types and constituents, pharmacists are equipped to make appropriate recommendations for infant formulas. For proper development, infant formulas should contain water, carbohydrates, protein, and fat in the recommended amounts (TABLE 2). Most infant formulas currently available in the United States are iron-fortified, containing approximately 12 mg of iron per liter. The AAP recommends that formula-fed infants receive an iron-fortified formula as a way of reducing the prevalence of iron-deficiency anemia. Pharmacists can also ensure that caregivers receive appropriate counseling on proper preparation, handling, and storage of infant formulas, in an effort to prevent the spread of infection (TABLE 3). Because support is critically important, knowing which resources to share with parents is essential for helping them achieve an optimal outcome for their infant.16-18
Vitamin and Mineral Supplementation
Vitamin D: All infants require vitamin D supplementation to prevent rickets, and this is particularly important for an infant whose mother is deficient in vitamin D. The vitamin D content of breast milk is constitutively low, and it is even lower in mothers with dark skin or other causes of vitamin D deficiency. In the U.S., milk, infant formula, breakfast cereals, and some other foods are fortified with vitamin D. Healthy term infants require 400 IU (10 mcg) of vitamin D daily, and those who are exclusively breastfed—as well as some formula-fed infants—require vitamin D supplements to achieve this target. Children and adolescents aged 1 year to 18 years require 600 IU (15 mcg) of vitamin D daily. These dosages are designed to maintain 25-hydroxyvitamin D levels >20 ng/mL (50 nmol/L) in most populations. It is important to note that, within a given population, individuals with certain risk factors may require higher dosages than those listed here.16,19-22
Iron: The minimum daily requirement (MDR) for iron varies depending on gestational age and birthweight. The MDR is 1 mg/kg for full-term infants and 2 mg/kg to 4 mg/kg for premature or low-birthweight infants. After age 4 months, the iron requirement for a full-term breastfed infant may exceed the amount supplied by human milk alone. In addition to human milk, some form of iron supplementation (e.g., pureed meats, iron-fortified infant cereal, iron-rich vegetables, liquid iron supplement) is recommended to provide at least 1 mg/kg per day. From 7 to 12 months of age, iron intake should be 11 mg per day. In general, an average of two servings of iron-fortified cereal in combination with human milk (a total of 30 g or one-half cup of dry cereal) is sufficient to meet the daily iron requirement. As complementary foods are introduced, those higher in iron should be offered early. When parents inquire about vitamin supplementation for their child, the pharmacist can encourage them to read product labels and carefully verify the serving size and percent daily value of iron in the product. Infants who receive iron-fortified formula do not need additional iron supplementation.16,23-26
Vitamin B12: The adequate intake of vitamin B12 is 0.4 mcg/day for infants from birth to age 6 months and 0.5 mcg/day for those aged 7 to 12 months. Vitamin B12 supplementation is recommended for breastfed infants of strict vegan mothers if the mother is not taking supplemental vitamin B12 while lactating, and for formula-fed infants who receive a strictly vegan complementary diet. The adverse neurologic consequences of vitamin B12 deficiency may be devastating and irreversible. Pharmacists can review with these parents the physiologically active vitamin B12 available in supplements and some fortified cereals, soy beverages, and nutritional yeast.16,27,28
Fat-Soluble Vitamins: Supplementation with vitamins A, D, E, and K is usually considered only for infants who have chronic cholestatic liver disease or fat malabsorption.23,29
Complementary Foods
The primary goal of feeding during the child’s first year is to obtain sufficient nutrients for optimal growth. Secondary goals include the acquisition of oromotor skills and the development of appropriate eating behaviors, as feeding practices established during the first 2 years of life lay the foundation for lifelong eating patterns. Solid foods should not be introduced until the infant can sit with support and has good head and neck control, which usually happens at age 4 to 6 months. As solid foods are introduced, formula or human milk should be given concurrently and gradually reduced as the child nears the first birthday, as this will provide the full range of nutrients needed for growth, development, and health (TABLE 2).1,2,16,23
Single-ingredient foods should be introduced first, and it is appropriate to offer one new food every 3 to 4 days to see how the child responds (i.e., any type of reaction). The AAP Committee on Nutrition suggests that infant cereals and pureed meats be offered first because they provide iron and zinc, which are the nutrients most likely to be deficient in the diets of U.S. infants. Pharmacists can educate parents about the importance of offering pureed meats, which provide heme iron (heme iron is more bioavailable than nonheme iron and can increase the absorption of nonheme iron). Additionally, pharmacists should recommend that at least one feeding per day contain foods rich in vitamin C in order to promote iron absorption. Once these foods are accepted, additional strained or pureed fruits and vegetables may be added to ensure that the energy and nutrient content are adequate (TABLE 2). Because infants need fat and cholesterol—particularly for optimal neurodevelopment—the intake of these foods usually is not restricted. If new parents feel discouraged about their child’s refusal of certain foods, the pharmacist can tell them that the acceptability of new foods increases with repeated exposure; in fact, it may take up to 15 exposures before a new food is accepted by the child.16,23,26,30-32
Certain foods should not be given to infants aged younger than 1 year. These include hard, round foods (e.g., nuts, grapes, raw carrots), which can lead to choking, and honey, which is associated with infant botulism. In addition, the AAP recommends avoiding unmodified cow’s milk in infants aged younger than 12 months because of the increased renal-solute load and the increased risk of iron deficiency. Additionally, home-prepared spinach, beets, green beans, squash, and carrots should not be given to infants aged younger than 4 months, as these foods may contain sufficient nitrate to cause methemoglobinemia. Finally, the addition of salt and sugar is discouraged, as is the consumption of commercial juice, as they provide no nutritional benefit and have adverse outcomes for children. The pharmacist should inform parents that adding sugar and salt does not increase the infant’s acceptance of foods and that avoiding added sugar and salt during infancy may help set a lower threshold for sweet and salty preferences later in life.23,33-37
Introducing Potentially Allergenic Foods
Delaying the introduction of foods considered to be highly allergenic beyond age 4 to 6 months was previously suggested as a way to prevent atopic disease in high-risk children (those with a first-degree relative with documented allergic disease). However, various professional groups, including the AAP Committee on Nutrition and Section on Allergy and Immunology and the European Society for Paediatric Gastroenterology Hepatology and Nutrition, found no convincing evidence that this practice has a significant protective effect. Allergenic foods such as eggs, fish, peanuts, and tree nuts may be introduced to infants aged 4 to 6 months even if the child is at risk for allergic disease, provided that the choking hazard is addressed. It is critical for pharmacists to counsel parents on the importance of medication readiness and the appropriate use, safety, and dose of an antihistamine (e.g., Benadryl) for a milder reaction, or, if medically indicated and for more severe reactions, an autoinjector (e.g., EpiPen).38-40
The Pharmacist’s Role
The role of the pharmacist in education and support for successful breastfeeding and the introduction of complementary foods and supplements has become increasingly important, along with the use of appropriate resources. Pharmacists serve a critical role in their communities as advocates for and supporters of optimal nutrition for child development. Prioritizing public-health policies that promote the provision of adequate nutrients, necessary vitamin supplementation, and healthy eating during this crucial time in a child’s life will ensure that all children have an early foundation for optimal development, a key factor in long-term health. The AAP website provides a wealth of material, links to other organizations, and other resources to assist and support pharmacists in their role as advocates for children’s well-being.1,2,11,41
REFERENCES
1. Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatr. 2012;129:e827.
2. American Academy of Family Physicians. Breastfeeding, family physicians supporting (position paper). www.aafp.org/about/policies/all/breastfeeding-support.html. Accessed April 17, 2020.
3. ACOG Committee Opinion No. 756: optimizing support for breastfeeding as part of obstetric practice. Obstet Gynecol. 2018;132:e187-e196.
4. World Health Organization. Breastfeeding. www.who.int/topics/breastfeeding/en. Accessed April 12, 2020.
5. Critch JN, Canadian Paediatric Society; Nutrition and Gastroenterology Committee. Nutrition for healthy term infants, six to 24 months: an overview. Paediatr Child Health. 2014;19:547-552.
6. Rodriguez-Palmero M, Koletzko B, Kunz C, Jensen R. Nutritional and biochemical properties of human milk: II. Lipids, micronutrients, and bioactive factors. Clin Perinatol. 1999;26:335-359.
7. Andreas NJ, Kampmann B, Le-Doare KM. Human breast milk: a review on its composition and bioactivity. Early Hum Dev. 2015;91:629-635.
8. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. 2013;60:49-74.
9. Garofalo R, Chheda S, Mei F, et al. Interleukin-10 in human milk. Pediatr Res. 1995;37:444-449.
10. Fituch CC, Palkowetz KH, Goldman AS, Schanler RJ. Concentrations of IL-10 in preterm human milk and in milk from mothers of infants with necrotizing enterocolitis. Acta Paediatr. 2004;93:1496-1500.
11. American Academy of Pediatrics. Breastfeeding initiatives. www.aap.org/breastfeeding. Accessed May 7, 2020.
12. Hale TW. Medication and Mother’s Milk. 15th ed. Amarillo, TX: Hale Publishing; 2012.
13. Briggs GG, Freeman RK, Yaffe SJ, eds. Drugs in Pregnancy and Lactation. 9th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2011.
14. Drugs and Lactation Database; 2012. http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?LACT.
15. Micromedex 2.0 [intranet database]. Greenwood Village, CO: Thomson Healthcare; 2020.
16. Institute of Medicine. Dietary reference intakes: the essential guide to nutrient requirements. www.nap.edu/catalog/11537.html. Accessed April 27, 2020.
17. Enfamil. Preparing your baby’s bottle. www.enfamil.com/app/iwp/enf10/content.do?dm=enf&id=/Consumer_Home3/NewBorns/NwBorns_Articles/preparingbottle&iwpst= B2C&ls=0&csred=1&r=3477143060. Accessed May 21, 2020.
18. Seattle Children’s Hospital. Bottle-feeding (formula) questions. www.seattlechildrens.org/conditions/a-z/bottle-feeding-formula-questions. Accessed May 21, 2020.
19. Misra M, Pacaud D, Petryk A, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics. 2008;122:398-417.
20. Braegger C, Campoy C, Colomb V, et al. Vitamin D in the healthy European paediatric population. J Pediatr Gastroenterol Nutr. 2013;56:692-701.
21. Wagner CL, Hulsey TC, Fanning D, et al. High-dose vitamin D3 supplementation in a cohort of breastfeeding mothers and their infants: a 6-month follow-up pilot study. Breastfeed Med. 2006;1:59-70.
22. Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101:394-415.
23. Fewtrell M, Bronsky J, Campoy C, et al. Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2017;64:119-132.
24. Baker RD, Greer FR, Committee on Nutrition American Academy of Pediatrics. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0-3 years of age). Pediatrics. 2010;126:1040-1050.
25. Calvo EB, Galindo AC, Aspres NB. Iron status in exclusively breast-fed infants. Pediatrics. 1992;90:375-379.
26. Walter T, Dallman PR, Pizarro F, et al. Effectiveness of iron-fortified infant cereal in prevention of iron deficiency anemia. Pediatrics. 1993;91:976-982.
27. Johnson PR Jr, Roloff JS. Vitamin B12 deficiency in an infant strictly breast-fed by a mother with latent pernicious anemia. J Pediatr. 1982;100:917-919.
28. von Schenck U, Bender-Götze C, Koletzko B. Persistence of neurological damage induced by dietary vitamin B-12 deficiency in infancy. Arch Dis Child. 1997;77:137-139.
29. Fomon SJ, Filer J Jr, Anderson TA, Ziegler EE. Recommendations for feeding normal infants. Pediatrics. 1979;63:52-59.
30. Krebs NF, Westcott JE, Butler N, et al. Meat as a first complementary food for breastfed infants: feasibility and impact on zinc intake and status. J Pediatr Gastroenterol Nutr. 2006;42:207-214.
31. Engelmann MD, Davidsson L, Sandström B, et al. The influence of meat on nonheme iron absorption in infants. Pediatr Res. 1998;43:768-773.?
32. Fildes A, Lopes C, Moreira P, et al. An exploratory trial of parental advice for increasing vegetable acceptance in infancy. Br J Nutr. 2015;114:328-336.
33. Hopkins D, Emmett P, Steer C, et al. Infant feeding in the second 6 months of life related to iron status: an observational study. Arch Dis Child. 2007;92:850-854.
34. American Academy of Pediatrics Committee on Nutrition: the use of whole cow’s milk in infancy. Pediatrics. 1992;89:1105-1109.
35. Ziegler EE, Fomon SJ. Potential renal solute load of infant formulas. J Nutr. 1989;119(suppl 12):1785-1788.
36. Recommendations to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47:1-29.
37. Greer FR, Shannon M, American Academy of Pediatrics Committee on Nutrition, American Academy of Pediatrics Committee on Environmental Health. Infant methemoglobinemia: the role of dietary nitrate in food and water. Pediatrics. 2005;116:784-786.
38. Agostoni C, Decsi T, Fewtrell M, et al. Complementary feeding: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2008;46:99-110.
39. Greer FR, Sicherer SH, Burks AW, Committee on Nutrition, Section on Allergy and Immunology. The effects of early nutritional interventions on the development of atopic disease in infants and children: the role of maternal dietary restriction, breastfeeding, hydrolyzed formulas, and timing of introduction of allergenic complementary foods. Pediatrics.2019;143:e20190281.
40. Salter SM, Delfante B, deKlerk S, et al. Pharmacists’ response to anaphylaxis in the community (PRAC): a randomised, simulated patient study of pharmacist practice. BMJ Open. 2014;4:e005648.
41. Ronai C, Taylor JS, Dugan E, Feller E. The identifying and counseling of breastfeeding women by pharmacists. Breastfeed Med. 2009;4:91-95.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





