US Pharm. 2024;49(11):17-26.
ABSTRACT: Diabetic eye disease, most commonly presenting as diabetic retinopathy, is the leading cause of preventable blindness worldwide. The pathogenesis of diabetic eye disease involves complex biochemical pathways that are set in motion by chronic hyperglycemia. Primary treatment options for diabetic retinopathy include laser photocoagulation and intravitreal injection of antivascular endothelial growth factor agents. Treatments aim to stabilize or improve visual function, but significant vision loss can still occur. Pharmacists play a vital role in supporting patients with the disease.
The most common diabetic eye disease is diabetic retinopathy (DR), a neurovascular disorder characterized by retinal damage.1 The leading cause of preventable blindness worldwide, DR develops in 75% of people with diabetes within 15 years of disease.1-3 As of 2021, 9.6 million people in the United States were estimated to have DR, and 1.84 million of these cases were vision-threatening.4 These numbers are expected to grow, with a projected increase of nearly threefold between 2005 and 2050.5
STRUCTURE AND FUNCTION OF THE EYE
The eye is composed of three layers: outer (cornea and sclera), intermediate (iris and choroid), and internal (retina). Three chambers are also present, two of which contain aqueous and one vitreous humor. The outer layer consists of the sclera and the cornea. Most of the eye is surrounded by a strong, white layer of protective tissue referred to as the sclera.6 The cornea is a clear, dome-shaped structure covering the anterior portion that regulates and focuses light.6
In the anterior and posterior chambers located behind the cornea, aqueous fluid is continuously produced and drained to maintain consistent intraocular volume and pressure. This fluid is produced by the ciliary body and drained through the trabecular meshwork, Schlemm’s canal, and the uveoscleral outflow pathway.6 Between these chambers, the iris contracts and expands by changing the size of the pupil, thus regulating the amount of light that passes through to the lens.6 This light is then bent or refracted by the lens and travels through the vitreous chamber to reach the retina.7
The retina is a thin tissue consisting of neuronal and glial cells that is considered part of the central nervous system.7,8 The retina converts received light into neurochemical signals for transport to the brain via the optic nerve.7 A tiny, specialized portion of the retina called the macula is responsible for detailed, central vision.6 The choroid is a highly vascularized structure located between the retina and the sclera. Blood flow to the retina is regulated by changes in systemic, intraocular, and perfusion pressure as well as tissue and metabolic factors.7
PATHOPHYSIOLOGY OF DIABETIC EYE DISEASE
Sustained hyperglycemia results in significant metabolic abnormalities that can result in damage to not only the retina and macula but also the optic nerve, lens, and many other parts of the eye.5 Many biochemical pathways contribute to the development of diabetic eye disease. Decreased perfusion and changes to the endothelial surface layer occur even in the early stages of diabetes.7
Retinal ischemia increases the expression of growth factors such as vascular endothelial growth factor (VEGF) and insulin-like growth factor 1.9 In diabetes, excess glucose is metabolized through the polyol pathway, leading to accumulation of intracellular sorbitol, inducing osmotic damage within retinal cells.9 Increased activation of protein kinase C influences several other pathways, resulting in changes in endothelial permeability, retinal hemodynamics, and increased activation and adhesion of leukocytes.9,10 Further, increased availability of glucose accelerates the formation of advanced glycation end products, which interact with various receptors to induce oxidative stress and inflammation.8,9 Neurodegeneration of retinal neurons and glial cells has recently been identified to contribute to the pathogenesis of DR.8,9
DIABETIC EYE DISEASE CLASSIFICATION
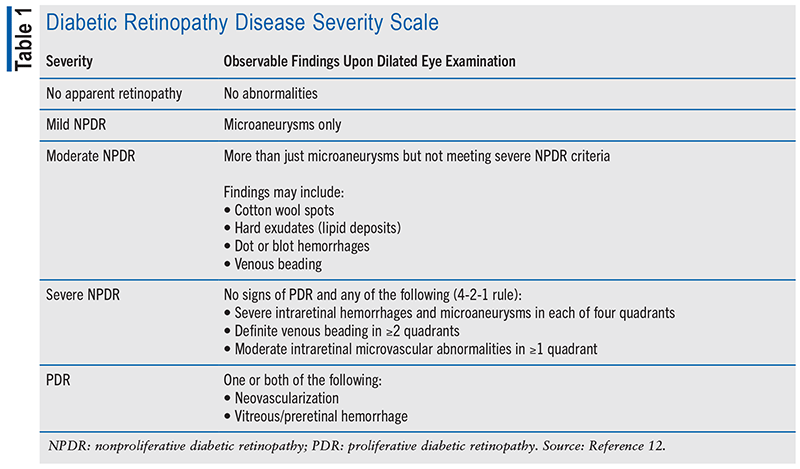
DR is categorized according to the degree of progression, initially without angiogenesis in nonproliferative DR (NPDR) and increasing in severity to proliferative DR (PDR). PDR is marked by the development of leaky and fragile blood vessels in the optic disc or elsewhere within the retina. Adjacently formed fibrous vessels stay behind as the blood vessels cycle through proliferation and regression, creating traction across the retina. Penetration and leakage of these vessels into the vitreous can lead to vitreous hemorrhage and retinal detachment.7 If left untreated, PDR confers a 50% chance of blindness within 5 years.11 Classifications of DR disease severity and observable findings are detailed in TABLE 1.
In any stage of DR, fluid can accumulate in the macula, resulting in diabetic macular edema (DME).12 DME has a prevalence of up to 71% of patients with PDR. Vision loss is most common in PDR and DME.11 The term clinically significant macular edema (CSME) is often used when retinal thickening (edema) and/or lipid deposits (hard exudates) involve or threaten to involve the center of the macula. As the risk of visual loss is greatest if DME is at the center of the macula, DME is subdivided into center-involved (CI-DME) and noncenter-involved (NCI-DME). Risk of visual loss is higher if the edema is located at the center of the macula.12
RISK FACTORS
The primary risk factor for the development of diabetic eye disease is high blood glucose over time.12 An elevated glycosylated hemoglobin A1C (HbA1C) increases the risk for development of DR, DME, and cataract. Risk of DR also rises with longer duration of diabetes. Additional risk factors include hypertension and dyslipidemia.3 The maintenance of near-normal levels of blood glucose and blood pressure reduces the risk of both development and progression of disease.12
TREATMENT
Timely screening, early detection, and appropriate follow-up can reduce the risk of severe vision loss from diabetic eye disease by 95%.1 Individuals with diabetes who have not been diagnosed with DR should be screened regularly. A dilated eye examination is recommended at the time of diagnosis (type 2) or 5 years after diagnosis (type 1) and annually thereafter.1
The primary goals of DR therapy are to improve or stabilize visual function and improve vision-related quality of life.12 Both the 2024 American Diabetes Association Standards of Care in Diabetes and the 2019 American Academy of Ophthalmology (AAO) Preferred Practice Patterns stress the importance of implementing strategies to help patients with diabetes reach glycemic, blood pressure, and lipid goals to reduce the risk or slow the progression of DR.1,12
Tight blood glucose control is the most critical factor in both delaying the onset and slowing the progression of DR.7 Intensive diabetes management resulting in near-normal blood glucose has been demonstrated to prevent and/or delay the onset and progression of DR, reduce the need for future ocular surgical procedures, and potentially improve visual function in large, prospective, randomized studies.1
Primary treatment options recommended in the 2019 AAO Preferred Practice Patterns include laser photocoagulation and intravitreal injection of anti-VEGF agents, with the support of vitrectomy and other pharmacotherapeutic agents. Specific recommendations are dependent on diagnosis, disease staging, and visual acuity.12
Laser Photocoagulation
The therapeutic effects of laser therapy targeting retinal tissue are caused by the absorption of light by ocular pigments, causing purposeful destruction of a fraction of photoreceptors.13 This is thought to reduce overall oxygen demand and retinal hypoxia, thereby decreasing upregulation of growth factor (including VEGF) production and increasing oxygen perfusion to the remaining viable retinal cells.7,13 The procedure can be performed in ambulatory settings.13
Laser photocoagulation can be applied specifically to a limited area in a focal laser approach or diffusely to peripheral retinal tissue in panretinal photocoagulation (PRP). Laser photocoagulation has been demonstrated to reduce the risk of moderate vision loss in DME and is recommended for the treatment of both CI-DME and NCI-DME.12 PRP is recommended for reduction in risk of vision loss in patients with high-risk PDR and in some cases severe NPDR.1 Visual side effects including light halos, glare, and poor night vision and permanent visual-field loss may occur in association with laser photocoagulation. Anatomic complications such as choroidal effusions, retinal detachments, and new-onset macular edema have also been reported.14
Vitrectomy
Vitrectomy is a surgical procedure involving the removal of the vitreous gel from the eye.14 It is used for significant vision-threatening complications such as vitreous hemorrhage or tractional retinal detachment.14 In patients with substantial vitreomacular traction who prove refractory to anti-VEGF agents and photocoagulation, vitrectomy may improve visual acuity.12 Results are variable (no benefit to great gains in visual acuity).12 Possible complications include retinal detachment, suprachoroidal hemorrhage, endophthalmitis, hypotony, cataract, and vision loss.12,13
Pharmacotherapy
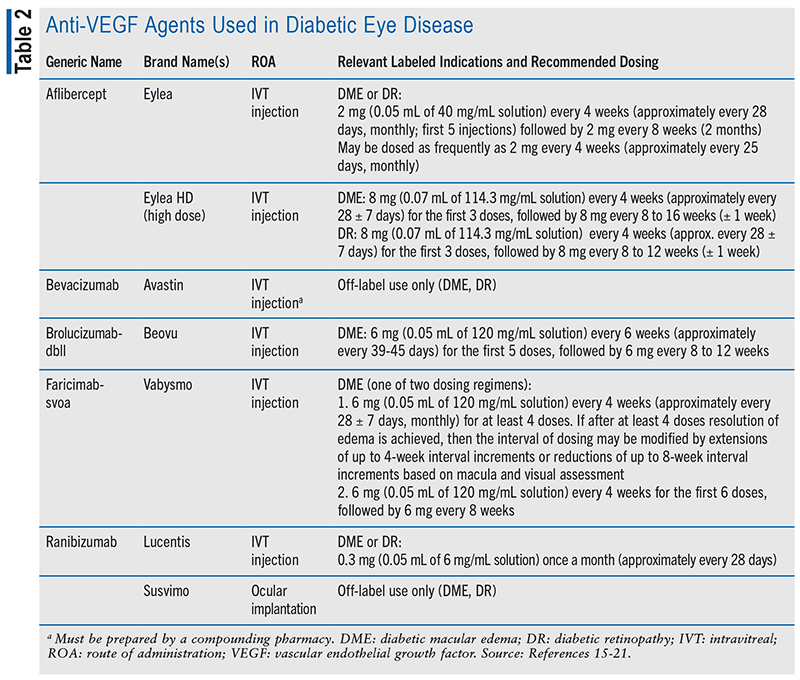
Anti-VEGF Therapy: Intravitreal injections of anti-VEGF agents are first-line treatment for CI-DME with impaired visual acuity (see TABLE 2).1,12 Multiple high-quality clinical trials have demonstrated this therapy to be more effective in improving vision in CI-DME than monotherapy with focal laser treatment.12 Intravitreal injections of anti-VEGF agents are a reasonable alternative to PRP for some patients with PDR.1 Several high-quality clinical trials demonstrated this therapy to be more effective than focal laser treatment in improving vision for this indication.12 After 2 years of follow-up, intravitreal injections of anti-VEGF agents have been found effective at regressing PDR and lead to noninferior or superior visual acuity outcomes compared with PRP.1
As a procedure, intravitreal injection increases the risk of transient increases in intraocular pressure (IOP) as well as injection-related infectious endophthalmitis.14 Risks of intravitreal injection of anti-VEGF agents specifically include retinal detachment, cataract, vitreous hemorrhage, uveitis, ocular inflammation, floaters, and retinal-vessel changes.14
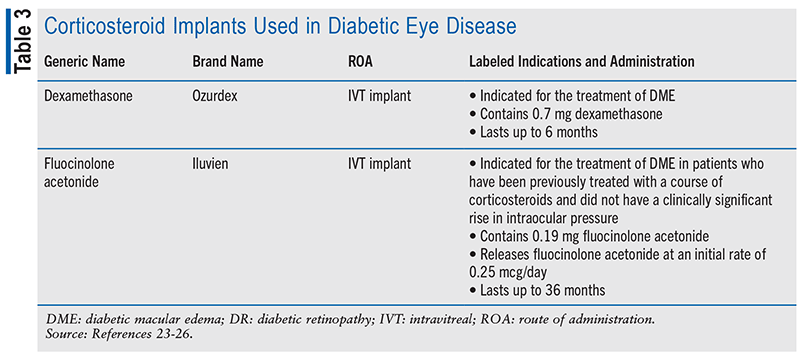
Corticosteroids: Intravitreal corticosteroids are considered second-line agents for DME due to a high risk of side effects, including cataract formation, cataract progression, and elevation of IOP. They are generally less effective, with only transient benefits that are outweighed by these risks.12 Intravitreal administration of corticosteroids, however, has been shown to improve visual acuity and edema to some extent.22 In addition to injectable liquid formulations such as dexamethasone and triamcinolone acetonide, which are often used off-label for the treatment of DME, implantable formulations are also available, as detailed in TABLE 3. Single-use, preloaded applicators are used to facilitate injection of these implants directly into the vitreous for sustained drug release over time. Topical and periocular steroid injections have demonstrated no benefit.12
RELATED EYE DISEASES
Advanced DR can lead to neovascular glaucoma (NVG) through the development of new vessels over the iris and fibrovascular tissue in the anterior chamber angle. NVG is sometimes referred to as diabetic hemorrhagic glaucoma.27 Increased IOP in NVG results in damage to the optic nerve and is often accompanied by substantial vision loss.27,28 PRP is the gold standard for treatment of NVG, although surgical procedures, drainage implants, and shunts may also be used.29 Pharmacotherapeutic options include intravitreal injection of anti-VEGF agents to decrease angiogenesis; various topical agents such as beta-blockers, carbonic anhydrase inhibitors, alpha agonists, and prostaglandin analogues to lower IOP; and topical atropine and corticosteroids for management of pain and inflammation.29
Sustained hyperglycemia also increases the risk of cataract, an opacification of the lens of the eye. The incidence of cataracts in the diabetic population is three to five times higher and occurs at an earlier age compared with the general population.30 Decreased translucency of the lens, which can be partial or complete, reduces visual acuity.30 Cataract pathogenesis is not fully understood but appears to be multifactorial. Oxidative stress, impaired autophagy, metabolic-composition changes in the aqueous humor, and dietary influences are thought to contribute.30,31 The main treatment method is surgical removal of the cataract with intraocular lens implantation.30
THE PHARMACIST’S ROLE
Achieving optimal vision outcomes and quality of life can be challenging for patients with diabetic eye disease. Treatments often require repeated specialist visits and are costly. Due to the correlation between HbA1C and disease progression, consistent and appropriate management of diabetes and other underlying disease states is key. Alongside surgical and pharmacologic therapies, patients with DR should be encouraged to maintain a healthy diet and lifestyle.12
Pharmacists involved in the outpatient care of this population can help improve outcomes by identifying possible roadblocks. The importance of regular follow-up should be emphasized. Substantial vision loss may still occur despite optimal therapy. Referrals for counseling, vision rehabilitation, or social services may be indicated according to specific patient needs.12
Treatment with anti-VEGF agents often requires regular injections and monitoring. Available anti-VEGF agents vary in their recommended dosing intervals, and the selection of a medication with greater spacing may improve therapeutic adherence.
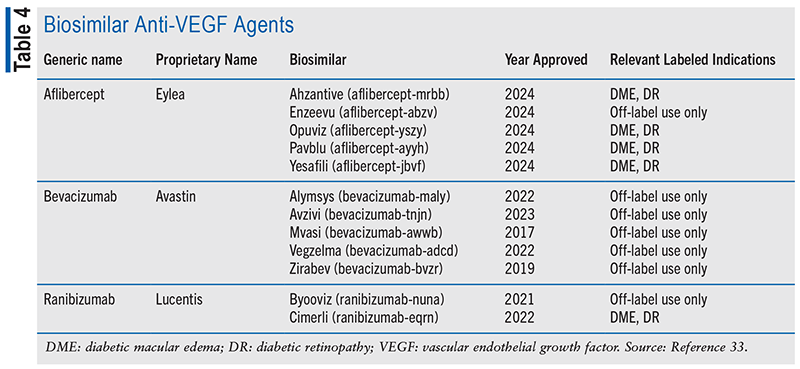
Biological therapies are generally more expensive than small-molecule medications. High cost may prevent patients from receiving the most effective treatment, resulting in poor treatment outcomes. Biosimilars are biological medicines that have no clinically meaningful differences from an approved reference drug but may have a lower cost. They undergo a rigorous analytical and clinical process to demonstrate their physicochemical and clinical similarity.32 Several biosimilar medicines have received FDA approval in recent years, although not all are yet available. Several of these choices are listed in TABLE 4.
CONCLUSION
Diabetic eye disease remains a significant global cause of preventable blindness, with its prevalence set to increase. Effective management of DR relies on timely screening, maintenance of glycemic control, and consistent ongoing treatment. Pharmacists play a critical role in supporting patient adherence to these therapies, especially as new options such as biosimilars become available.
REFERENCES
1. American Diabetes Association Professional Practice Committee. Retinopathy, neuropathy, and foot care: Standards of Care in Diabetes—2024. Diabetes Care. 2024;47(Suppl 1):s231-s243.
2. Tan TE, Wong TY. Diabetic retinopathy: looking forward to 2030. Front Endocrinol (Lausanne). 2022;13:1077669.
3. Fung TH, Patel B, Wilmot EG, Amoaku WM. Diabetic retinopathy for the non-ophthalmologist. Clin Med (Lond). 2022;22(2):112-116.
4. CDC. Prevalence estimates for diabetic retinopathy (DR). May 15, 2024. www.cdc.gov/vision-health-data/prevalence-estimates/dr-prevalence.html. Accessed August 10, 2024.
5. National Institute of Diabetes and Digestive and Kidney Diseases. Diabetic eye disease. www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/diabetic-eye-disease. Accessed August 3, 2024.
6. American Academy of Ophthalmology. Eye anatomy: parts of the eye and how we see. April 29, 2023. www.aao.org/eye-health/anatomy/parts-of-eye. Accessed August 18, 2024.
7. Wright WS, Eshaq RS, Lee M, et al. Retinal physiology and circulation: effect of diabetes. Compr Physiol. 2020;10(3):933-974.
8. Zhou J, Chen B. Retinal cell damage in diabetic retinopathy. Cells. 2023;12:1342.
9. Tarr JM, Kaul K, Chopra M, et al. Pathophysiology of diabetic retinopathy. ISRN Ophthalmol. 2013;2013:343560.
10. Li H, Liu X, Zhong H, et al. Research progress on the pathogenesis of diabetic retinopathy. BMC Ophthalmol. 2023;23:372.
11. Lundeen EA, Burke-Conte Z, Rein DB, et al. Prevalence of diabetic retinopathy in the US in 2021. JAMA Ophthalmol. 2023;141(8):747-754.
12. Flaxel CJ, Adelman RA, Bailey ST, et al. Diabetic Retinopathy Preferred Practice Pattern. Ophthalmology. 2020;127(1):66-145.
13. Everett LA, Paulus YM. Laser therapy in the treatment of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2021;21(9):35.
14. Bahr TA, Bakri SJ. Update on the management of diabetic retinopathy: anti-VEGF agents for the prevention of complications and progression of nonproliferative and proliferative retinopathy. Life. 2023;13(5):1098.
15. Eylea (aflibercept) product information. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; December 2023.
16. Eylea HD (aflibercept) product information. Tarrytown, NY: Regeneron Pharmaceuticals, Inc; December 2023.
17. Avastin (bevacizumab) product information. San Francisco, CA: Genentech, Inc; September 2022.
18. Beovu (brolucizumab) product information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; July 2024.
19. Vabysmo (faricimab) product information. San Francisco, CA: Genentech, Inc; July 2024.
20. Lucentis (ranibizumab) product information. San Francisco, CA: Genentech, Inc; February 2024.
21. Susvimo (ranibizumab) product information. San Francisco, CA: Genentech, Inc; November 2022.
22. Tatsumi T. Current treatments for diabetic macular edema. Int J Mol Sci. 2023;24:9591.
23. Ozurdex (dexamethasone intravitreal implant) product information. Madison, NJ: Allergan USA, Inc; December 2022.
24. Ozurdex diabetic macular edema (DME) and treatment with Ozurdex patient education guide. https://hcp.ozurdex.com/content/dam/ozurdexhcp/pdf/US-OZU-230013_021100_OZX-DME-Patient-Slim-Jim_HR.pdf. Accessed October 3, 2024.
25. Iluvien (fluocinolone acetonide intravitreal implant) product information. Alpharetta, GA: Alimera Sciences, Inc; December 2021.
26. Alimera Sciences, Inc. Iluvien. https://hcp.iluvien.com. Accessed October 3, 2024.
27. Senthil S, Dada T, Das T, et al. Neovascular glaucoma—a review. Indian J Ophthalmol. 2021;69(3):525-534.
28. Glaucoma Research Foundation. The relationship between diabetes and glaucoma. November 2, 2023. www.glaucoma.org/articles/the-relationship-between-diabetes-and-glaucoma. Accessed August 20, 2024.
29. American Academy of Ophthalmology. Diagnosis and management of neovascular glaucoma. April 1, 2018. www.aao.org/eyenet/article/diagnosis-and-management-of-neovascular-glaucoma. Accessed August 18, 2024.
30. Mrugacz M, Pony-Uram M, Bryl A, Zorena K. Current approach to the pathogenesis of diabetic cataracts. Int J Mol Sci. 2023;24(7):6317.
31. Kiziltoprak H, Tekin K, Inanc M, Goker YS. Cataract in diabetes mellitus. World J Diabetes. 2019;10(3):140-153.
32. Hariprasad SM, Gale RP, Weng CY, et al. An introduction to biosimilars for the treatment of retinal diseases: a narrative review. Ophthalmol Ther. 2022;11(3):959-982.
33. FDA. Purple book: database of licensed biological products. https://purplebooksearch.fda.gov. Accessed August 18, 2024.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





