Diabetes is a condition that can affect adults and children and may lead to complications such as nephropathy, retinopathy, peripheral neuropathy, and atherosclerotic cardiovascular disease.1 Approximately 8.2% of the United States population was diagnosed with diabetes in 2018. Based on data collected from 2013 through 2016, 50% of U.S. adults with diabetes had a hemoglobin A1C (HbA1C) >7%. According to 2018 data, insulin therapy was initiated in approximately 10.9% of U.S. adults within 1 year of being diagnosed with diabetes. Populations with prevalence rates of >10% in the United States include Native Americans, Hispanic Americans, and African Americans.2

GLUCOSE MONITORING
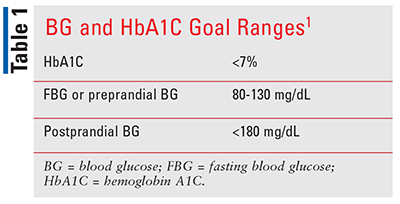
TABLE 1 summarizes recommendations for target blood glucose (BG) and HbA1C goals in adult patients with diabetes. The American Diabetes Association recommends that patients monitor glucose levels to aid in optimal management. One way to monitor glucose is by using a continuous glucose monitoring (CGM) system. Types of CGM systems are real-time CGM (RT-CGM) that automatically transmit glucose levels to the user, intermittently scanned CGM (IS-CGM) that require the user to scan the sensor to obtain glucose data, and integrated CGM (iCGM) that allow for integration with other compatible medical devices/electronic interfaces.1
THE DEXCOM G6 CGM SYSTEM
There are several CGM systems available for use. One example is the Dexcom G6 CGM System. Dexcom G6 is an integrated RT-CGM system indicated for use as a replacement for fingersticks in patients 2 years and older to make diabetes treatment decisions. Additionally, Dexcom G6 is the only iCGM system that alerts patients of impending hypoglycemia and offers customizable monitoring settings. Dexcom G6 is the only RT-CGM system that does not require scanning or fingersticks for initial calibration, insulin dosing, and treatment decisions (when the sensor code is entered). However, if glucose alerts and readings from Dexcom G6 do not match symptoms, patients should use a BG meter to make diabetes treatment decisions.3
Dexcom G6 received positive reviews from numerous patients. Approximately 92% of survey respondents indicated that Dexcom G6 is easy to use.4 Pharmacies may order the system through all major wholesalers.5 Additionally, three out of four commercially insured patients on mealtime insulin have coverage for Dexcom G6. One-third of patients paid zero out-of-pocket costs for their Dexcom G6 at the pharmacy.4
Features and Capabilities
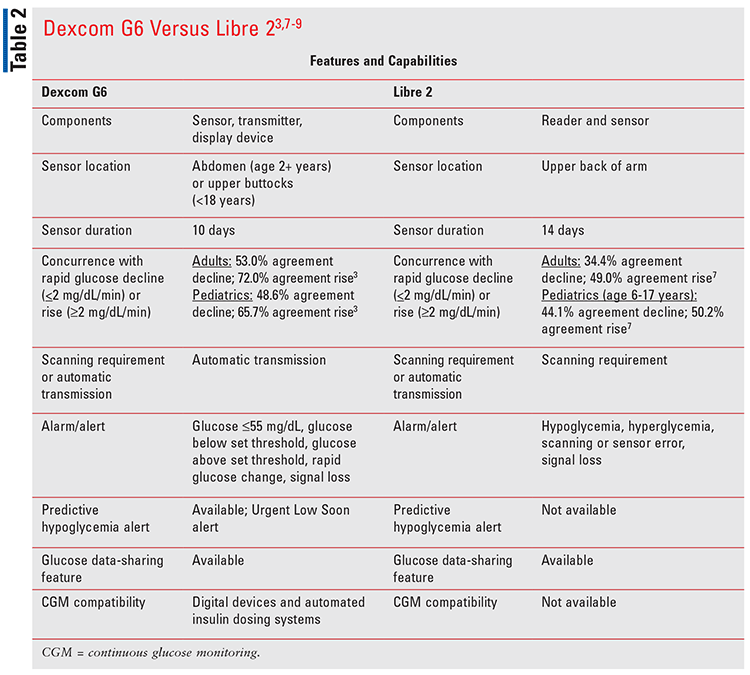
Dexcom G6 has several advantages. It is the only iCGM that has approval to work with other compatible insulin delivery devices and automated pumps.6,7 Dexcom G6 data is compatible with multiple mobile applications, making it easy for both patients and providers to monitor glucose levels. TABLE 2 compares features and capabilities of Dexcom G6 and Abbott’s FreeStyle Libre 2 Flash Glucose Monitoring System (Libre 2).
Dexcom G6 is the only iCGM that can detect and predict dangerous hypoglycemic episodes (i.e., glucose is 55 mg/dL or lower) 20 minutes before occurrence.3 When such episodes occur, an Urgent Low Soon alert warns the patient. To this end, a real-world study observed that the alert was associated with a significant reduction in severe hypoglycemia without causing a subsequent increase in hyperglycemia.10
Dexcom Share and the Dexcom Follow app are features that allow patients with Dexcom G6 to share glucose information with up to 10 followers.3 A study in pediatric patients (aged 6-12 years) discovered those who had at least one follower spent less time in hypoglycemia (1% vs. 1.3%) and hyperglycemia (20% vs. 23.9%) and used their Dexcom devices more regularly (6.2 days vs. 4.7 days). Approximately 95% of Dexcom pediatric users took advantage of the Share feature.11
Components and Software
There are three main components: the sensor, transmitter, and display device. The sensor is inserted under the skin via an autoapplicator. The sensor monitors glucose levels in the interstitial fluid. The transmitter must be attached to the sensor in order to wirelessly send data to the display device to be viewed. Glucose readings are displayed as frequently as every five minutes. Patients can use the receiver or a compatible smart device to view the data. FIGURE 1 shows the components included and compatible smart devices.3 Additionally, patients and providers have access to Dexcom CLARITY, which is a reporting tool that is available to providers over the web and to users through their smart device and the web. Users can share up to 90 days of glucose data with providers and can also receive insights on glycemic patterns from Dexcom CLARITY through push notifications via a smart device. Patients also have the option to receive push notifications from the device.12
Clinical Efficacy
Studies demonstrate that Dexcom CGM is clinically effective. One study discovered a statistically significant decrease in HbA1C levels (mean 1.0% A1C reduction, P <0.001) after 24 weeks in patients with type 1 diabetes who used Dexcom CGM.13 The authors also conducted a study in patients with type 2 diabetes who used Dexcom CGM and obtained substantive results: within 24 weeks of monitoring, HbA1C levels were reduced by 0.8% (P = 0.022).14
A study demonstrated that patients who used Dexcom CGM had a decreased probability of prolonged episodes of hypoglycemia when compared with users who used intermittent glucose-monitoring devices.15
Compared to periods of Dexcom G5 use, Dexcom G6 use was associated with statistically significant reductions in the frequency of hypoglycemia and hyperglycemia and improvements in time spent within the target glucose range.16
Patient Support
Patients who require assistance using Dexcom G6 have access to Dexcom CARE, a training program that helps users become familiar with features of the device. There are other online tutorials and training videos available to patients. Additionally, Dexcom CARE provides Certified Diabetes Care and Education Specialists for live assistance.17 Commercially insured patients may be eligible to save up to $140 in copays on Dexcom G6 through the patient voucher program by visiting dexcom.com/pharmacyoffer. Another program is Dexcom Start, which helps simplify the prescribing process for HCPs.4
CONCLUSION
Dexcom G6 has been shown to be beneficial for patients who require CGM. As outlined in this article, the system has several features that make it advantageous for use in clinical settings. More information about Dexcom G6 is available at the following video link: provider.dexcom.com/information-pharmacists.
Dexcom, Dexcom G6, Dexcom Share, Dexcom Follow, Dexcom CLARITY, and Dexcom CARE are registered trademarks of Dexcom, Inc. in the United States and/or other countries. ©2021 Dexcom, Inc. All rights reserved. LBL021672 Rev 001
REFERENCES
1. American Diabetes Association Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Suppl 1).2. CDC. National Diabetes Statistics Report, 2020. www.cdc.gov/diabetes/data/statistics-report/index.html. Published August 28, 2020. Accessed March 31, 2021.
3. Dexcom G6 CGM User Guide, 2020.
4. Dexcom, data on file, 2020.
5. Dexcom, Inc. Dexcom G6 Continuous Glucose Monitoring (CGM) System. Dexcom, Inc. https://provider.dexcom.com/sites/default/files/document/2020-10/toolkit_for_pharmacists_0.pdf. Accessed April 2, 2021.
6. FDA. 510(k) Premarket Notification: Dexcom G6 Continuous Glucose Monitoring System. www.accessdata.fda.gov/cdrh_docs/reviews/K200876.pdf. Accessed April 2, 2021.
7. FDA. 510(k) Premarket Notification: FreeStyle Libre 2 Flash Glucose Monitoring System. www.accessdata.fda.gov/cdrh_docs/reviews/K193371.pdf. Accessed April 2, 2021.
8. Abbott Libre 2 User Guide, 2020.
9. Abbott Diabetes Care, Inc. LibreLinkUp FAQs. www.librelinkup.com/faqs. Accessed August 5, 2021.
10. Puhr S, Derdzinski M, Welsh JB, et al. Real-world hypoglycemia avoidance with a continuous glucose monitoring system’s predictive low glucose alert. Diabetes Technology & Therapeutics. 2019;21(4):155-158.
11. Welsh JB, Derdzinski M, Parker AS, et al. Real-time sharing and following of continuous glucose monitoring data in youth. Diabetes Therapy. 2019;10(2):751-755.
12. Dexcom CLARITY. Dexcom, Inc. https://provider.dexcom.com/products/dexcom-clarity. Accessed April 2, 2021.
13. Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections. JAMA. 2017;317(4):371-378.
14. Beck RW, Riddlesworth TD, Ruedy K, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections. Annals of Internal Medicine. 2017;167(6):365-374.
15. Reddy M, Jugnee N, El Laboudi A, et al. A randomized controlled pilot study of continuous glucose monitoring and flash glucose monitoring in people with type 1 diabetes and impaired awareness of hypoglycemia. Diabetic Medicine. 2018;35(4):483-490.
16. Welsh JB, Gao P, Derdzinski M, et al. Accuracy, utilization, and effectiveness comparisons of different continuous glucose monitoring systems. Diabetes Technology & Therapeutics. 2019;21(3):128-132.
17. Dexcom, Inc. Dexcom CARE. www.dexcom.com/dexcom-care. Published December 10, 2020. Accessed April 2, 2021.





