US Pharm. 2011;36(5):HS-9-HS-14.
Opioids have long been considered a cornerstone of effective therapy for pain management. However, the prescribing of opioids has been associated with apprehension and contentious debate in the health care community, which has created barriers to achieving adequate pain relief. Fear of opioid abuse, misuse, addiction, and diversion taint the reputation and potential benefits of opioid narcotics. Current statistics regarding illicit drug use support this apprehension: The 2009 National Survey on Drug Use and Health (NSDUH) revealed that 8.7% of the population aged 12 years and older used some type of illicit drug.1 Of these, 32% used prescription medications—including pain relievers, tranquilizers, stimulants, and sedatives—for nonmedical use.1
Opioid Use in Pain Management
While the use of some illicit drugs (i.e., cocaine and methamphetamines) is declining in young adults, the use of prescription drugs is on the rise. From 2002 to 2009, there was a significant increase—from 5.5% to 6.3%—in the nonmedical use of prescription medications in adults aged 18 to 25 years.1 This was due primarily to an increase in the misuse of pain relievers.1 The NSDUH found that more than half of all pain relievers were obtained from a friend, the majority of whom obtained the prescription from one physician; 17.6% of drug users obtained the pain reliever directly from their own physician.1 Only 4.8% of pain relievers were obtained through an illicit supplier.1
Patients who use narcotics therapeutically for pain relief are at risk for accidental overdose. The CDC’s National Center for Injury Prevention and Control released a brief describing unintentional drug poisoning through 2007. Trends toward unintentional drug overdose have steadily increased every year, with 27,658 unintentional overdoses occurring in 2007.2,3 Opioid narcotics were the leading cause of overdose, surpassing cocaine and heroin deaths combined.2,3 Middle-aged patients (age 45 to 54 years) demonstrated the highest rates of unintentional drug overdose.2
These trends support concerns regarding the safe and legal use of opioid narcotics for pain management and have driven the consideration of risk-management strategies that ensure that access to narcotics for therapeutic use remains available and also promote efforts to stem misuse and overdose.3
Background on REMS
Under the purview of the FDA Amendments Act of 2007, the FDA has the authority to enforce postmarketing risk-management strategies.4 Accordingly, the FDA has mandated that manufacturers develop risk evaluation and mitigation strategies (REMS) for selected drugs that may require additional safeguards in order for the benefit of use to outweigh the risk. The REMS program is an expansion of previous risk-management programs enacted by the FDA, including medication guides and risk-minimization action plans.4 Manufacturers seeking FDA approval for new drugs may be required to submit REMS proposals, and drugs already on the market also may be required to develop REMS. A drug is considered misbranded if it is not prescribed and dispensed in accordance with the required REMS, and violations of REMS may be subject to monetary penalties.4
Currently, REMS have been approved for more than 150 drugs.5 Frequently, REMS are necessitated by adverse effects associated with the agent. Approximately one-quarter of all original new drug approvals in 2010 had REMS, indicating the growing utilization and impact of the REMS program.6 Additionally, the FDA may review drugs that currently have medication-guide requirements or other risk-management strategies in order to determine whether REMS should be developed.4
REMS are not uniform; they differ depending upon the type of risk management required. REMS may include the following components: medication guides, communication plans, elements to assure safe use (ETASUs), and implementation systems. Medication guides, which are geared toward the patient, help ensure that the patient understands the risks and adverse effects associated with the drug.4 Requirements for the distribution of medication guides may vary with each specific drug. Communication plans target the hospital, pharmacy director, or prescriber and promote the safe use and prescribing of these agents. Fewer agents are associated with ETASUs, which entail active elements that hospitals or prescribers must adhere to. Examples of ETASUs include prescriber–patient agreements, voluntary and mandatory education plans, and prescriber enrollment programs. Implementation systems primarily include manufacturer monitoring and audits of outcomes and hospital and prescriber compliance.
REMS’ Current Impact on Opioids
Given the statistics underscoring the increased misuse of opioid narcotics, coupled with the alarming rates of unintentional overdose, opioids are a clear example of a drug class that requires the use of risk-management strategies. Such strategies may help ensure that health care providers and patients are aware of appropriate dosing, safety considerations, and risks associated with inappropriate use. The goal of REMS is to allow access to these agents while assuring that the benefits of use outweigh the risks.3 Currently, nine opioid narcotics already have REMS (TABLE 1).5 Notably, many of these agents have unique characteristics, such as transdermal administration or extended-release formulation, that increase the risk of abuse and misuse.
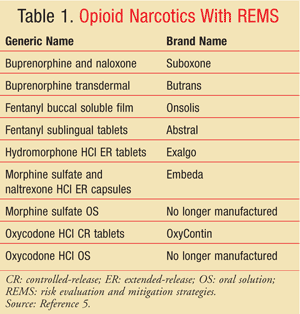
Similar to nonopioid REMS, the REMS associated with the aforementioned opioids are not uniformly fashioned. Morphine sulfate oral solution requires a medication guide to be dispensed, whereas hydromorphone extended-release tablets require the dispensing of a medication guide and ETASUs (health care provider letter, health care professional education training kit, and registration).5,7 Buprenorphine and naloxone sublingual film requires the dispensing of a medication guide and ETASUs (verification of diagnostic criteria, patient counseling, physician and pharmacist brochure, and appropriate-use checklist).8 Furthermore, buprenorphine and naloxone film requires an implementation system that monitors compliance with the ETASUs. The fentanyl buccal soluble film contains all possible components of REMS: a medication guide, a communication plan, ETASUs, and an implementation system. The manufacturer maintains a program utilizing patient enrollment, prescriber certification and enrollment, and limited dispensing from pharmacies enrolled in the program. The manufacturer’s implementation system involves verification of prescriber and pharmacy enrollment in the program, database maintenance, monitored distribution, and audits of pharmacies and wholesalers.9
The educational components specific to opioid REMS attempt to educate the prescriber and the patient on a multitude of factors. Patients are cautioned to guard opiates to prevent theft or diversion by others; they are also warned of the dangers of using opioids other than as directed by the prescriber or in combination with other central nervous system depressants.7 Prescribers are educated about safety considerations and abuse of prescriptions and are given suggestions for establishing clinically therapeutic dosing and ensuring patient compliance.7 Patient education occurs through medication guide distribution, counseling, and, in some cases, ETASUs. Education of prescribers and dispensers occurs through communication letters and ETASUs.
Classwide REMS
The concept of classwide REMS has already been recognized. For the oncologic use of erythropoietin-stimulating agents (ESAs), the ESA APPRISE (Assisting Providers and cancer Patients with Risk Information for Safe use of ESAs) program went into effect on February 16, 2011. This classwide program involves prescriber training and enrollment in the ESA APPRISE database; patient counseling and education; signing and maintenance of a patient–prescriber agreement acknowledging risks associated with ESA use; medication-guide distribution; and audits of manufacturer compliance.10
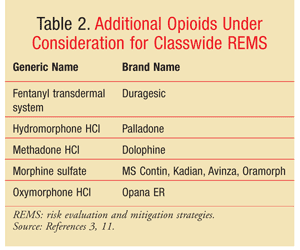
The FDA is currently considering the enforcement of classwide REMS for long-acting and extended-release opioids. In addition to the opioids that already have REMS, this classwide REMS mandate would impact both the brand and generic formulations of agents such as morphine and hydromorphone.3 The brand and generic agents affected are listed in TABLE 2.3,11 Because the exact specifications of the program are still under deliberation, it is not clear what the REMS will entail.
The FDA has conducted numerous meetings with health care professionals, pain specialists, patients, and industry members to solicit feedback and discuss the proposed opioid REMS. In July 2010, a joint meeting of the Anesthetic and Life Support Drugs Advisory Committee and the Drug Safety and Risk Management Advisory Committee was convened to discuss the proposed classwide opioid REMS. This board voted 25 to 10 against the FDA’s proposed classwide opioid REMS, primarily because of the majority’s belief that the program was not restrictive enough.12 In lieu of voluntary prescriber education, as proposed by the FDA, the board believed that education should be mandated. The committee also thought that the FDA should oversee and develop an educational program and develop metrics for evaluating the success of the REMS. Further recommendations by the board included mandatory patient counseling and ETASUs such as registration or documentation, and in a clear directive foreshadowing the continued expansion of REMS, the board noted that it wished this classwide REMS to be applicable to immediate-relief formulations as well.12,13
Impact on Hospitals
The impact of REMS on hospitals is only now starting to be realized. Foremost, the multifaceted components of REMS necessitate the institution of administrative systems. Resources are needed to organize prescriber enrollment, maintain documentation, disseminate educational materials, and ensure appropriate patient counseling. Although the manufacturer develops the REMS, the ultimate responsibility for enforcing it falls to the hospital, the prescriber, and the pharmacy. Furthermore, hospitals face the potential for increased opioid-acquisition costs passed along by manufacturers, who will be required to spend additional resources for developing REMS materials, enforcing documentation, and ensuring compliance.
Hospital administration must organize a system to manage the numerous components of REMS, such as determining which areas necessitate distribution of medication guides and identifying proper documentation and storage of patient–prescriber agreements. Hospitals and pharmacists must devise storage strategies for medication guides, which are associated with costs such as production and printing. The hospital must develop a system to disseminate information about the REMS requirements and subsequent updates or new additions. This is a difficult task, as drugs continue to be added, removed, or modified according to REMS requirements.
The Pharmacist’s Role
The pharmacist is one of many on the health care team who will be affected by the demands of REMS. Pharmacists in inpatient, outpatient, management, and community settings alike will be affected by REMS requirements. Pharmacists must ultimately ensure that drugs dispensed are in compliance with all of the elements of REMS. As such, hospital pharmacists may be required to verify prescriber enrollment in manufacturer programs or ensure completion of educational training. Such safeguards will require dedicated training of pharmacists regarding the goals of REMS. Pharmacists must realize the importance of properly verifying and enforcing such safeguards.
Pharmacists may work in conjunction with various health care providers to ensure the dispensing of medications guides and patient–prescriber agreement forms, when necessary. They may also be directly involved in counseling patients about medications. Pharmacists who practice on clinical inpatient floors or in outpatient dispensing pharmacies or clinics are well positioned to further reiterate that safety is paramount and precautions must be taken when using opioids. Additionally, pharmacists can assess patient comprehension of the materials and counseling received and help evaluate pain management. In outpatient settings, pharmacist diligence and recordkeeping may help prevent abuse, falsification of prescriptions, and diversion.
As the final step in the chain before a product is dispensed, it is imperative that pharmacists familiarize themselves with the specifications of each REMS requirement to confirm that all necessary steps have been completed. Appropriate management of a patient’s pain, coupled with adherence to the guidelines created by REMS, is necessary to optimize the safe and appropriate use of opioids. Diligent provision of pharmaceutical care in this manner will ensure hospital compliance, patient comprehension, and safety.
The Future of REMS
Trends regarding continuous REMS development for existing drugs, as well as new agents, suggest that REMS will continue to become a more prominent aspect of drug administration and dispensing. Hospitals and pharmacies alike should develop policies and strategies for accommodating new REMS in order to streamline their continued growth and expansion.
Prescribers and pharmacists should remain updated on existing and developing REMS and should take the increased emphasis on safety as a reminder of the many risks associated with certain drugs.14 Opioid narcotics should be used with caution and care, and a balance must be struck between providing adequate pain relief and assuring adequate safeguards. Many critics of REMS suggest that the programs hinder access to health care and place an unnecessary burden on the hospital system. While REMS may increase barriers to prescribing, proper accommodations for these changes by pharmacists can help minimize these challenges and ensure that access and quality of pain management continue. The profession of pharmacy embodies a commitment to the safety and well-being of patients, and pharmacists should embrace REMS as an effort to enhance patient care.
REFERENCES
1. Substance Abuse and Mental Health Services Administration. Results From the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. Rockville, MD: Office of Applied Studies, NSDUH Series H-38A, HHS Publication No. SMA 10-4586Findings; 2010.
2. CDC. CDC’s issue brief: unintentional drug poisoning in the United States.
www.cdc.gov/
3. FDA. Background on opioid REMS.
www.fda.gov/Drugs/DrugSafety/
4. FDA. Guidance for industry: format and content of proposed risk evaluation and mitigation strategies (REMS), REMS assessments, and proposed REMS modifications.
www.fda.gov/downloads/Drugs/
5. FDA. Approved risk evaluation and mitigation strategies (REMS).
www.fda.gov/Drugs/DrugSafety/
6. FDA. Drug approval reports: original new drug approvals by month.
www.accessdata.fda.gov/
7. Exalgo (hydromorphone HCl extended-release) package insert. Hazelwood, MO: Mallinckrodt Brand Pharmaceuticals, Inc; March 2010.
8. Suboxone (buprenorphine and naloxone) package insert. Richmond, VA: Reckitt Benckiser Pharmaceuticals Inc; September 2010.
9. Onsolis (fentanyl buccal soluble film) package insert. Somerset, NJ: Meda Pharmaceuticals, Inc; July 2009.
10. ESA APPRISE Oncology Program.
www.esa-apprise.com. Accessed January 20, 2011.
11. Egervary A. FDA looking to add REMS for certain opioids.
www.pharmacist.com/AM/
12. FDA. Summary minutes of the joint meeting of the Anesthetic and Life Support Drugs Advisory Committee (ALSDAC) and the Drug Safety and Risk Management Advisory Committee (DSaRM). July 22-23, 2010.
www.fda.gov/downloads/
13. Yap D. FDA proposes REMS for certain opioids. Pharmacy Today [pain suppl]. 2001;S11-S12.
14. Ready for REMS. As a healthcare provider, how do I prepare for opioid risk evaluation and mitigation strategies (REMS)?
http://readyforrems.com/pdf/
To comment on this article, contact rdavidson@uspharmacist.com.





