US Pharm. 2023;48(8):HS2-HS10.
ABSTRACT: Community-acquired pneumonia (CAP) is the most common infectious cause of hospitalizations and deaths in children. The etiology of CAP in hospitalized children is typically viral, with respiratory syncytial virus being the most common pathogen in children aged <2 years. Streptococcus pneumoniae is the chief bacterial cause of CAP in children aged <5 years. Hallmark signs include fever, cough, poor feeding, and hypoxemia. Positive chest radiography is informative for assessing clinical outcome and disease etiology, which helps guide disease management. CAP in hospitalized patients should be managed according to current treatment guidelines, and the pharmacist should ensure that antimicrobial stewardship and preventive measures are implemented.
Each year, pneumonia affects an estimated 226 million children aged <5 years worldwide.1 It is also the leading cause of infectious disease–related hospitalizations and deaths in children. The World Health Organization stated that more than 740,000 deaths have occurred annually in this patient population.1 Community-acquired pneumonia (CAP) is an acute infection of the lung parenchyma that an individual contracts in a community setting. Mortality rates for pneumonia in the United States and other countries can be lowered by implementing advanced management measures and treatment guidelines.1 However, death rates are higher in children with comorbidities, such as chronic lung disease and congenital heart disease, and in those receiving immunosuppressive therapy.2,3
Epidemiology and Etiology
In 2011, the Pediatric Infectious Disease Society and the Infectious Diseases Society of America (PIDS/IDSA) established guidelines for the management of pediatric CAP.3 It is important to conduct a visual inspection of the patient. It is recommended that all children hospitalized for CAP undergo chest radiography upon admission to document the presence and extent of pulmonary infiltrates and identify complications. Results of chest radiography are informative for assessing the etiology and prognostic outcome.3 Studies have identified several factors that can aid in determining CAP severity, and ongoing research is examining the correlation between chest radiograph, time of clinical stability of temperature, heart rate, respiratory rate, and supplemental oxygen use.2,4
Microbiological identification is another a key determinant in the prognostic evaluation of the CAP patient. Although many microorganisms can cause CAP, viral and bacterial sources are most common in previously healthy children. The etiologic organisms responsible for pneumonia vary greatly according to the patient’s age. The etiology of CAP in hospitalized children is typically viral in nature, respiratory syncytial virus (RSV) being the most common pathogen in children aged <2 years. Mycoplasma pneumoniae is the most virulent causative microorganism leading to severe CAP in children. Group B streptococcus is a leading cause of pneumonia in newborns, and Streptococcus pneumoniae is the chief cause of pyogenic bacterial pneumonia in children aged <5 years; however, S pneumoniae does not lead to severe CAP.4
Diagnostic Criteria
Hallmark signs of CAP in children include fever, cough, poor feeding, and hypoxemia. Children with sustained oxygen saturation of <90% on room air and/or other signs of respiratory distress, such as tachypnea, dyspnea, retractions, grunting, nasal flaring, apnea, or altered mental status, should be hospitalized for treatment of CAP.3 Infants and children with suspected methicillin-resistant Staphylococcus aureus (MRSA) CAP and any infant aged <3 months to 6 months with suspected bacterial CAP should also be hospitalized.3
Children aged <5 years and those with comorbidities are at increased risk for severe pneumonia and therefore should be considered for hospitalization.3,5,6 Presumptive noncompliance or concerns about lack of follow-up may warrant hospitalization to reduce the risk of disease progression. Other factors, such as inability to take oral medications, vomiting, or dehydration, may also prompt the consideration of hospitalization for CAP.3
Clinical Management
Since S pneumoniae is the most common causative pathogen of bacterial CAP in children, empiric treatment targeting this pathogen is usually employed.1,3,7,8 In children aged <5 years, CAP is frequently caused by respiratory viruses; therefore, antimicrobial therapy is not routinely used unless a bacterial source is suspected.3 The treatment of CAP in children may be divided into two categories: outpatient management and inpatient management.
Outpatient Management: Amoxicillin is the recommended first-line treatment for mild-to-moderate CAP in children who are immunized and otherwise healthy.3 Atypical pathogens may be responsible for some cases of CAP in children aged >5 years with atypical pneumonia symptoms (sore throat, headache, cough, low-grade fever), slow progression of symptoms over 3 to 5 days, and/or nonfocal auscultatory and chest x-ray findings.9,10 In these instances, macrolides are considered first-line treatment for mild-to-moderate CAP. Macrolides should not be used empirically outside of suspected atypical bacterial CAP because of the higher incidence (~40%) of macrolide resistance in strains of community S pneumoniae.3 In patients who have experienced a nonsevere allergic reaction to amoxicillin, treatment options include a trial of amoxicillin or cephalosporins with susceptibility against S pneumoniae, such as cefpodoxime, cefprozil, or cefuroxime, with the selected option administered under medical supervision.3 In patients with a severe allergic reaction to amoxicillin, alternative treatment options include respiratory fluoroquinolones, linezolid, and, if susceptible, macrolides or clindamycin.3
Inpatient Management: Treatment for bacterial CAP is determined based on whether a patient has been fully immunized against Haemophilus influenzae and S pneumoniae; also, IV treatment is prioritized over oral treatment. The routine immunization of children with the H influenzae type B conjugate vaccine has essentially eradicated H influenzae, and only those with chronic lung disease or obstruction might still develop H influenzae CAP.3 Therefore, ampicillin or penicillin G is recommended as first-line therapy in fully immunized children. In children who are not fully vaccinated, reside in an area with a high incidence of penicillin resistance in S pneumoniae isolates, or present with life-threatening pneumonia, a third-generation cephalosporin is recommended as first-line therapy.3 A macrolide is recommended in addition to a cephalosporin when M pneumoniae or Chlamydia pneumoniae is considered in the patient’s differential diagnosis. Vancomycin is indicated as adjunctive therapy to a beta-lactam only when there is clinical suspicion of MRSA. See TABLE 1 for a summary of empiric treatments for pediatric bacterial CAP.
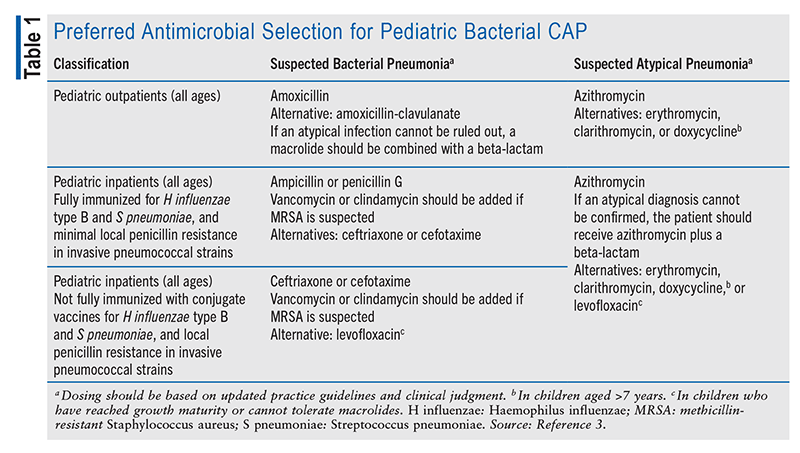
For hospitalized children who are fully immunized and have a penicillin allergy, a third-generation cephalosporin (ceftriaxone or cefotaxime) is the recommended alternative treatment option. In patients who are bacteremic, caution should be taken when alternative treatments to penicillin are used, given the heightened risk of meningitis.3 In children who are not fully immunized or who have an allergy to cephalosporins, respiratory fluoroquinolones are recommended as an alternative.3
Therapy Duration: Clinical response to antibiotic therapy is usually demonstrated within 48 to 72 hours of treatment. Hospitalized children who demonstrate clinical improvements in fever, appetite, and activity level for >12 to 24 hours and also maintain a pulse oximetry >90% may be evaluated for deescalation of IV antibiotics to oral alternatives for discharge. The standard duration of therapy for pediatric CAP is 10 days, but recent studies have found that children with nonsevere CAP have similar response rates with a 5-day course; this results in less antibiotic exposure, thereby potentially reducing the incidence of antibiotic resistance.3,11,12 A longer therapy duration may be warranted in patients with MRSA CAP or severe CAP, including complications such as pleural effusion, empyema, or lung abscess.3 In these instances, treatment may extend past 10 days—up to 4 to 6 weeks, depending on disease severity.3
To prevent antibiotic overuse, biomarkers such as procalcitonin may be used to monitor clinical response to therapy and to prevent the use of antibiotics for nonbacterial CAP. Procalcitonin rapidly increases within 3 to 6 hours of a bacterial infection and can peak at 8 to 24 hours, whereas the level remains near stable with viral infections, making this a viable means of avoiding the use of antibiotics for viral CAP.13 Because procalcitonin also increases with disease progression, it could be an indicator of clinical worsening or improvement of CAP.14 A recent meta-analysis found that procalcitonin reduced antibiotic duration by approximately 2 days and significantly reduced adverse effects associated with antibiotic use.15
The Pharmacist’s Role
The potential for severe complications and poor patient outcomes can be reduced with the participation of a pharmacist on an interdisciplinary team (IDT) and in an antimicrobial-stewardship program. The role of the pharmacist in managing hospitalized children with CAP is multifaceted and can lead to the improved use of antibiotics. Ensuring that patients are given guideline-recommended empiric antibiotic regimens that can be appropriately escalated or deescalated based on patient-specific factors can help lessen the incidence of multidrug-resistant organisms.16
Through immunization advocacy, pharmacists can help prevent future infections.17 As a result, pharmacist intervention has the potential to improve health outcomes in children hospitalized for CAP.3,16,17 Immunization advocacy is the key preventive-care intervention pharmacists can employ in an IDT setting. According to the 2011 PIDS/IDSA clinical practice guidelines, immunizations are the primary recommended prevention method for minimizing the risks associated with CAP in children.3 Additionally, it is recommended that patients be immunized for diseases that may cause or exacerbate an existing case of pneumonia infection, such as S pneumoniae, H influenzae type B, pertussis, and influenza.3 Also, infants at high risk for RSV should be immunized with palivizumab.3
Pharmacists must remain knowledgeable about recent immunization recommendations and approvals for appropriate CAP coverage, as updates occur frequently. For example, the pneumococcal conjugate vaccine (PCV), which covers several types of S pneumoniae, is available in three approved formulations: PCV13, PCV15, and PCV20.18 Although PCV20 is not currently recommended as a CAP immunization for children, its clinical efficacy and newly approved status may result in some updated recommendations in the future.19,20
Pharmacists can educate providers, patients, and caregivers about available vaccines and when they should be administered, and they can assist hospitalized patients in receiving needed immunizations prior to discharge. As drug-information experts, they can provide evidence-based recommendations that are both individualized and generally beneficial for patients.3,21 Therefore, pharmacists are vital to ensuring that antibiotics are used appropriately and that proper preventive-care measures are implemented in hospitalized children with CAP.
Conclusion
CAP is a highly prevalent and preventable cause of hospitalization in children. The use of patient-specific visual inspection, chest radiography, and microbiological identification results in a definitive diagnosis and identification of the causative agent. Factors such as age, pathogen, antibiotic exposure, immunization status, and therapy setting determine the empiric treatment of choice. As key members of the IDT, pharmacists play an important role in optimizing antibiotics, through appropriate use and dosage, to decrease antibiotic resistance. In the outpatient setting, pharmacists can counsel patients or caregivers on antibiotic use as well as utilize immunization advocacy to help prevent CAP in children.
REFERENCES
1. World Health Organization. Pneumonia in children. www.who.int/news-room/fact-sheets/detail/pneumonia. Accessed May 28, 2023.
2. McClain L, Hall M, Shah SS, et al. Admission chest radiographs predict illness severity for children hospitalized with pneumonia. J Hosp Med. 2014;9(9):559-564.
3. Bradley JS, Byington CL, Shah SS, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-e76.
4. Jain S, Williams DJ, Arnold SR, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015;372(9):835-845.
5. United States Census Bureau. Statistical Abstract of the United States 2008: The National Data Book. 127th ed. Washington, DC: United States Government Printing Office; 2008:159.
6. Tan TQ, Mason EO Jr, Barson WJ, et al. Clinical characteristics and outcome of children with pneumonia attributable to penicillin-susceptible and penicillin-nonsusceptible Streptococcus pneumoniae. Pediatrics. 1998;102(6):1369-1375.
7. Juvén T, Mertsola J, Waris M, et al. Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr Infect Dis J. 2000;19(4):293-298.
8. Wubbel L, Muniz L, Ahmed A, et al. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr Infect Dis J. 1999;18(2):98-104.
9. Kutty PK, Jain S, Taylor TH, et al. Mycoplasma pneumoniae among children hospitalized with community-acquired pneumonia. Clin Infect Dis. 2019;68(1):5-12.
10. Luby JP. Pneumonia caused by Mycoplasma pneumoniae infection. Clin Chest Med. 1991;12(2):237-244.
11. Williams DJ, Creech CB, Walter EB, et al. Short- vs standard-course outpatient antibiotic therapy for community-acquired pneumonia in children: the SCOUT-CAP randomized clinical trial. JAMA Pediatr. 2022;176(3):253-261.
12. Pernica JM, Harman S, Kam AJ, et al. Short-course antimicrobial therapy for pediatric community-acquired pneumonia: the SAFER randomized clinical trial. JAMA Pediatr. 2021;175(5):475-482.
13. Samsudin I, Vasikaran SD. Clinical utility and measurement of procalcitonin. Clin Biochem Rev. 2017;38(2):59-68.
14. Wacker C, Prkno A, Brunkhorst FM, Schlattmann P. Procalcitonin as a diagnostic marker for sepsis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(5):426-435.
15. Li P, Liu J, Liu J. Procalcitonin-guided antibiotic therapy for pediatrics with infective disease: a updated meta-analyses and trial sequential analysis. Front Cell Infect Microbiol. 2022;12:915463.
16. Puzz L, Plauche EA, Cretella DA, et al. Evaluation of a pediatric community-acquired pneumonia antimicrobial stewardship intervention at an academic medical center. Antibiotics (Basel). 2023;12(4):780.
17. Queeno BV. Evaluation of inpatient influenza and pneumococcal vaccination acceptance rates with pharmacist education. J Pharm Pract. 2017;30(2):202-208.
18. CDC. Pneumococcal vaccination: what everyone should know. www.cdc.gov/vaccines/vpd/pneumo/public/index.html. Accessed May 24, 2023.
19. FDA. Prevnar 20. www.fda.gov/vaccines-blood-biologics/vaccines/prevnar-20. Accessed May 24, 2023.
20. Senders S, Klein NP, Lamberth E, et al. Safety and immunogenicity of a 20-valent pneumococcal conjugate vaccine in healthy infants in the United States. Pediatr Infect Dis J. 2021;40(10):944-951.
21. Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955-964.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






