US Pharm. 2016;41(9):HS2-HS6.
ABSTRACT: A uterine transplant is a nonessential procedure that is undertaken to enable a woman with a damaged, underdeveloped, or absent uterus to bear children. Since 2000, 12 patients worldwide have had a uterine transplant, and seven pregnancies and four live births have been reported. Unique among transplants, the donated uterus is removed from the recipient after one or two successful births. Removal of the donated uterus eliminates the lifelong burden of immunosuppression, which may include medications such as tacrolimus, mycophenolate mofetil, azathioprine, and prednisone. The goals of pharmacotherapy are to prevent thrombosis in the transplanted organ’s blood vessels and to avoid infection during immunosuppression.
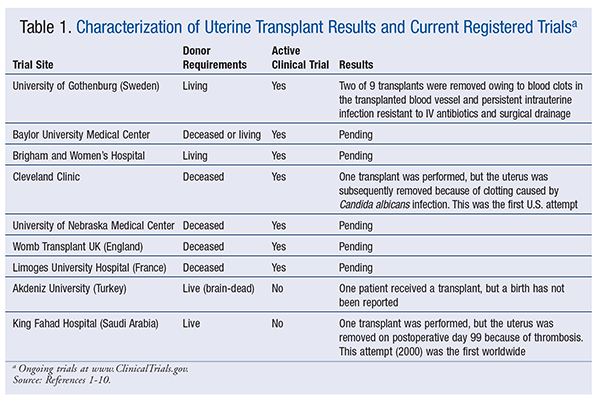
A uterine transplant is a nonessential procedure that is undertaken to enable a woman with a damaged, underdeveloped, or absent uterus to bear children. Since 2000, 12 women have undergone uterine transplants.1-4 Single transplants have been reported in Saudi Arabia, Turkey, and the Cleveland Clinic.1-3 Research at the University of Gothenburg in Sweden has resulted in nine transplants.4 In the United States, clinical trials are in progress at Baylor University Medical Center, Brigham and Women’s Hospital, the Cleveland Clinic, and the University of Nebraska Medical Center.5-8 Trials are also under way at sites in the United Kingdom and France.9,10 See TABLE 1.
The Recipient Population
One of every 4,000 women in the U.S. has Mayer-Rokitansky-Küster-Hauser syndrome, which is characterized by the congenital absence of the uterus and vagina.11-13 There are other situations that may prompt uterine transplant. One patient in Sweden had a uterine transplant after the loss of her uterus to cervical cancer.4 The first uterine transplant took place in Saudi Arabia in a patient who had lost her uterus 6 years earlier because of postpartum hemorrhage.1
The Donor Population
Transplants may be received from live or deceased donors. Surgery on deceased donors is simpler and carries no risk.14 However, the use of live donors allows greater control over the process and presumably delivers organs that are in better condition.15 Donor risks may extend beyond those assumed in surgery. Farrell and Falcone commented on the case of a donor whose uterus was involved in the first successful birth following a uterine transplant.15 Because the donor was postmenopausal, she was treated with combined oral contraceptives for 90 days prior to donation to optimize the uterine vasculature. Farrell and Falcone indicated that this method put the donor at greater risk for thromboembolic events before and immediately after the surgery.15
Harvesting
A patient who receives a uterine transplant will ultimately undergo in vitro fertilization. Egg harvesting involves the use of a gonadotropin-releasing hormone agonist (e.g., nafarelin), follicle-stimulating hormone (FSH), and human chorionic gonadotropin (hCG).16 In the Swedish study, blastocysts and embryos were harvested from the transplant recipient approximately 1 year before the anticipated date of transplant.16 The patient underwent two cycles of harvesting, which was initiated when the patient was in her luteal phase. The patient initially received intranasal nafarelin 400 mcg twice daily. Upon successful downregulation (defined previously as serum estradiol <50 pg/mL), the nafarelin dosage was reduced to 200 mcg for 14 days.16,17 Subsequently, FSH 175 IU was administered daily for 11 days to stimulate the patient’s ovaries.16 Finally, hCG alpha 250 mcg was administered SC to induce oocyte maturation. Venous and arterial thromboembolic events have been reported following the use of FSH.18 Because occlusions within transplanted uteri are the predominant source of failure, awareness of this risk is especially relevant to this patient population.
The Procedure
The Swedish researchers have published their procedure for transplanting a uterus.4 Acute pharmacotherapy is required during the surgery, and long-term immunosuppressive pharmacotherapy is necessary to maintain the viability of the transplanted uterus.
Before the transplant, recipients received mycophenolate mofetil 1 g, and both recipients and donors received piperacillin-tazobactam 4 g.4 To initiate immunosuppression, patients were given methylprednisolone 500 mg. To deplete T lymphocytes, patients also received two IV injections of antithymocyte globulin antibodies (either rabbit antithymocyte globulin 2.5 mg/kg or equine antithymocyte globulin 5 mg/kg) 12 hours apart.4
The anesthesia used in donors and recipients during their respective procedures was similar. A mixture of bupivacaine 10 mg and morphine 0.1 mg was given intrathecally at the L3-4 or L4-5 level. Anesthesia was induced with an infusion of remifentanil 0.25 mg/kg/min IV and a propofol bolus (2-3 mg/kg), which was followed with rocuronium 40 to 50 mg IV.4 Propofol is contraindicated in all patients with egg or soy allergy, despite a lack of evidence supporting this practice.19 After intubation, anesthesia was maintained with sevoflurane and remifentanil infusion.4 Ringer’s acetate (maximum 3 L) and dextran 70 (0.5 L) were given over the course of the operation.4 To maintain fluid balance, hydroxyethyl starch 500 mL or albumin 20 g was administered on an as-needed basis.4 IV dopamine was used to keep mean arterial pressure at a level of >65 mmHg.4
At the end of surgery, the surgical team administered ondansetron 4 to 8 mg, betamethasone 4 mg, and droperidol 0.5 to 1 mg IV to reduce postoperative nausea.4 Tacrolimus, droperidol, and ondansetron can independently prolong the QT interval.20-23
Post Transplant
Following the transplant, patients received maintenance immunosuppressive therapy consisting of tacrolimus (adjusted to trough levels of 10-15 ng/mL during wk 1-5 and 5-10 ng/mL during wk 6 and thereafter) twice daily and mycophenolate mofetil (with mycophenolate mofetil AUC trough levels of 40-60 mg • h/L) twice daily.4 Prednisone oral tablets were administered once daily on the day of surgery and during the first four postoperative days.4
Rejection Episodes
To detect the incidence of organ rejection, the Saudi study used the CD4/CD8 ratio and Doppler ultrasound to study flow volume, pulsatility, and the resistance index.1 On the ninth postoperative day, the patient reported low abdominal and back pain, general fatigue, malaise, and body ache accompanied by serosanguinous discharge, low-grade fever, and tachycardia. The CD4/CD8 ratio was elevated, and the Doppler scan detected myometrial edema. Increasing doses of cyclosporine and azathioprine and a single IV dose of methylprednisolone failed to treat the rejection. Two days after administration of antithymocyte globulin 2.5 mg/kg, signs of rejection were gone and the CD4/CD8 ratio improved.1
In the Swedish study, investigators took biopsies routinely and when graft rejection was suspected based on the following criteria: discolored cervix, abnormal vaginal discharge, enlarged uterus, fever, and/or abdominal pain.4 Nine rejection episodes were detected in five patients. All episodes were asymptomatic and diagnosed via biopsy.24 Eight episodes resolved after administration of methylprednisolone 500 mg IV once daily for 3 days followed by 7 days of oral prednisolone, and the ninth episode responded to oral glucocorticoids.24
Oocyte Implantation
To ensure that the transplant is stable, the implantation of embryos is performed 12 to 18 months after the uterine transplant takes place.24
Pregnancy
It has been suggested that kidney-transplant patients who are taking a calcineurin inhibitor (e.g., tacrolimus) increase the dosage during pregnancy in order to maintain optimal drug levels.25 In the Swedish study, tacrolimus, azathioprine, and prednisone were administered throughout pregnancy, and trough levels of 5 to 10 ng/mL were targeted, as previously described.4,16 One patient’s hemoglobin level declined from a median of 101 g/L to 79 g/L in week 18.16 Treatment with oral ferrous sulfate caused major gastrointestinal side effects, and investigators administered two subsequent IV doses of ferric carboxymaltose 500 mg, along with darbepoetin alfa 60 mcg weekly. The patient’s hemoglobin level increased to approximately 95 g/L.16
The Swedish protocol does not use mycophenolate mofetil during pregnancy because fetal risk was previously demonstrated with the use of this agent.4,26
Therapy Following the Birth
As of December 2015, four uterine-transplant patients have successfully given birth.27 In the Swedish study, tacrolimus was reduced by 45% the day after delivery.16
Unique among transplants, all uterine transplants are temporary; the donated organ is removed after the recipient has had one or two children, decreasing the burden of immunosuppressive medications on the recipient.4 At 2.5 months after giving birth, one transplant recipient decided to have her uterus removed; azathioprine was stopped, and a hysterectomy was performed 3.5 months after delivery.16 Tacrolimus was discontinued the day before surgery, and the oral prednisolone dosage was reduced by 50%—from 5 to 2.5 mg per day—and continued for 14 days.16
As with any hysterectomy, the pharmacist should be aware of the need for an adequate presurgical antibiotic regimen, the cessation of chronic medications, deep venous thrombosis prophylaxis, nausea treatment, and postoperative pain therapy.28 For more information on the pharmacist’s role in hysterectomy, see Reference 28.
Infection Risk
Of the procedures performed to date, two transplants have been removed because of infection-related complications. In both cases, an emergency hysterectomy was performed.3,4 The first patient in the Swedish study had a persistent intrauterine infection that was resistant to IV antibiotics and surgical drainage.4 The patient was readmitted on postoperative day 33 with abdominal pain, fever, and vaginal discharge, and cultures were positive for Enterococcus faecalis. Acute symptoms resolved after 4 days of unspecified IV antibiotics, but the patient remained on oral or IV antibiotics. On postoperative day 98, acute symptoms reemerged, and a CT scan revealed an intrauterine abscess that was resistant to surgical drainage. When signs of septicemia developed, a hysterectomy was performed.4 Candida albicans systemically infected the Cleveland Clinic patient, causing clotting that resulted in a hysterectomy.3 Future protocols may include the use of prophylactic antifungal medications.
The Swedish protocol administers antiviral prophylaxis during the transplant procedure.4 When valganciclovir and mycophenolate mofetil are taken concomitantly, plasma levels of both drugs may increase.29 Recipients and/or donors were given valganciclovir 450 mg for varying lengths of time based on detection of cytomegalovirus.4 Piperacillin-tazobactam administered on the day of the transplant was continued three times daily for 1 day in donors and three times daily for 3 days in recipients.4 One month after the uterus used in the second successful birth was removed, the patient developed an infected vaginal vault hematoma that was treated with unspecified oral antibiotics and transvaginal drainage to good effect.4
Thrombosis Risk
The patient in the Saudi study received Progyluton for 3 months following her uterine transplant to stimulate buildup of the atrophic endometrium.1 Progyluton, which is indicated as hormonal therapy for pre- and postmenopausal symptoms, is a calendar pack containing 11 tablets of estradiol valerate 2 mg and 10 tablets of estradiol valerate 2 mg plus norgestrel 0.5 mg that are taken sequentially; the next 7 days are tablet-free.30 As with other estrogen-containing products, Progyluton is presumed to increase the risk of thrombosis in postmenopausal women.30 On day 99, this patient experienced acute symptoms, including heavy vaginal discharge.1 Doppler scan showed the cessation of uterine blood flow, and a hysterectomy was performed immediately.1 A subsequent biopsy revealed uterine thrombosis.1 Prophylactic use of antithrombotic therapy was not mentioned in this report.1
Clotting was also a problem in the Swedish study. Three days after surgery, one patient demonstrated sudden cessation of the uterine-artery Doppler signal.4 Subsequent laparotomy showed an absence of pulses in the uterine arteries.4 After hysterectomy, focal necrosis, moderate ischemia, and arterial and venous occlusions were present.4 In this study, all recipients received dalteparin 5,000 IU on postoperative days 1 to 42 and aspirin 75 mg daily from time of transplant until graft removal. No other patients developed thrombosis requiring hysterectomy.4
Conclusion
Obstacles to uterine transplants encountered thus far include infection following immunosuppressive therapy and the development of thrombosis. However, the successful births in Sweden and the development of clinical trials at six additional sites in the U.S. and abroad offer hope that women with uterine factor infertility someday will be able to bear children.
REFERENCES
1. Fageeh W, Raffa H, Jabbad H, Marzouki A. Transplantation of the human uterus. Int J Gynaecol Obstet. 2002;76:245-251.
2. Ozkan O, Akar ME, Erdogan O, et al. Uterus transplantation from a deceased donor. Fertil Steril. 2013;100:e41.
3. Rubin R. Why the first American uterus transplant failed. Forbes. www.forbes.com/sites/ritarubin/2016/04/08/cleveland-clinic-doctors-say-an-infection-forced-them-to-remove-the-first-u-s-transplanted-uterus/#3bdc9ae8206d. Accessed May 23, 2016.
4. Brännström M, Johannesson L, Dahm-Kähler P, et al. First clinical uterus transplantation trial: a six-month report. Fertil Steril. 2014;101:1228-1236.
5. ClinicalTrials.gov. Uterine transplantation and pregnancy induction in women affected by absolute uterine infertility. https://clinicaltrials.gov/ct2/show/NCT02656550. Accessed July 28, 2016.
6. ClinicalTrials.gov. Uterine transplant in absolute uterine infertility (AUIF). https://clinicaltrials.gov/ct2/show/NCT02741102?term=Uterine+Transplant+in+Absolute+Uterine+Infertility+%28AUIF%29&rank=1. Accessed July 28, 2016.
7. ClinicalTrials.gov. Uterine transplantation for the treatment of uterine factor infertility. https://clinicaltrials.gov/ct2/show/NCT02573415?term=Uterine+Transplantation+for+the+Treatment+of+Uterine+Factor+Infertility&rank=1. Accessed July 28, 2016.
8. ClinicalTrials.gov. Initiation of a deceased donor uterine transplantation program at the University of Nebraska Medical Center. https://clinicaltrials.gov/ct2/show/NCT02409147?term=Initiation+of+a+Deceased+Donor+Uterine+Transplantation+Program+at+the+University+of+Nebraska&rank=1. Accessed July 28, 2016.
9. ClinicalTrials.gov. Human clinical trial of uterine transplantation in the United Kingdom. https://clinicaltrials.gov/ct2/show/NCT02388802?term=Human+Clinical+Trial+of+Uterine+Transplantation+in+the+United+Kingdom&rank=1. Accessed July 28, 2016.
10. ClinicalTrials.gov. Uterine allotransplantations using uterine grafts from brain-dead female donors (ATU). https://clinicaltrials.gov/ct2/show/NCT02637674?term=Uterine+allotransplantations+using+uterine+grafts+from+brain-dead+female+donors+%28ATU%29&rank=1. Accessed July 28, 2016.
11. Johannesson L, Järvholm S. Uterus transplantation: current progress and future prospects. Int J Womens Health. 2016;8:43-51.
12. Oppelt P, Renner SP, Kellermann A, et al. Clinical aspects of Mayer-Rokitansky-Kuester-Hauser syndrome: recommendations for clinical diagnosis and staging. Hum Reprod. 2006;21:792-797.
13. Pittock ST, Babovic-Vuksanovic D, Lteif A. Mayer-Rokitansky-Küster-Hauser anomaly and its associated malformations. Am J Med Genet A. 2005;135:314-316.
14. Tzakis AG. The first live birth subsequent to uterus transplantation. Transplantation. 2015;99:8-9.
15. Farrell RM, Falcone T. Uterine transplant: new medical and ethical considerations. Lancet. 2015;385:581-582.
16. Brännström M, Bokström H, Dahm-Kähler P, et al. One uterus bridging three generations: first live birth after mother-to-daughter uterus transplantation. Fertil Steril. 2016 Apr 25 [Epub ahead of print].
17. Borm G, Mannaerts B. Treatment with the gonadotrophin-releasing hormone antagonist ganirelix in women undergoing ovarian stimulation with recombinant follicle stimulating hormone is effective, safe and convenient: results of a controlled, randomized, multicentre trial. The European Orgalutran Study Group. Hum Reprod. 2000;15:1490-1498.
18. Follistim AQ Cartridge (follitropin beta injection) package insert. Whitehouse Station, NJ: Merck & Co, Inc; December 2014.
19. Asserhøj LL, Mosbech H, Krøigaard M, Garvey LH. No evidence for contraindications to the use of propofol in adults allergic to egg, soy or peanut. Br J Anaesth. 2016;116:77-82.
20. Prograf (tacrolimus) package insert. Northbrook, IL: Astellas Pharma US, Inc; May 2015.
21. Charbit B, Alvarez JC, Dasque E, et al. Droperidol and ondansetron-induced QT interval prolongation: a clinical drug interaction study. Anesthesiology. 2008;109:206-212.
22. Droperidol package insert. Shirley, NY: American Regent Laboratories Inc; January 2002.
23. Zofran (ondansetron injection) package insert. Research Triangle Park, NC: GlaxoSmithKline; September 2014.
24. Johannesson L, Kvarnström N, Mölne J, et al. Uterus transplantation trial: 1-year outcome. Fertil Steril. 2015;103:199-204.
25. Kim H, Jeong JC, Yang J, et al. The optimal therapy of calcineurin inhibitors for pregnancy in kidney transplantation. Clin Transplant. 2015;29:142-148.
26. CellCept (mycophenolate mofetil) package insert. South San Francisco, CA: Genentech USA, Inc; July 2015.
27. Brännström M. Uterus transplantation. Curr Opin Organ Transplant. 2015;20:621-628.
28. Bellanger R, Horlen C. Hysterectomy: what is the pharmacist’s role? US Pharm. 2011;36(9):HS4-HS7.
29. Valcyte (valganciclovir hydrochloride) package insert. South San Francisco, CA: Genentech USA, Inc; April 2015.
30. Progyluton (estradiol valerate and norgestrel) package insert. Weimar, Germany: Bayer Weimar GmBH & Co; July 2012.
To comment on this article, contact rdavidson@uspharmacist.com.






