US Pharm. 2007;32(12):66-67.
It is estimated that 10 to 20 million Americans suffer from neuropathy.1
Associated with various etiologies including diabetes, neurotoxic
chemotherapy, alcoholism, and nutrient deficiencies, neuropathy is a
microvascular syndrome that affects the autonomic, sensory, and motor neurons
of the peripheral nervous system. Neuropathy can be misdiagnosed or
undertreated and eventually results in neuropathic pain. Neuropathic pain,
which results from nerve cell dysfunction, is characterized by diminished
sensation, numbness or tingling in the extremities, deep-seated pain, or
increased sensitivity to pain. This malfunction can lead to upregulation of
sodium and calcium channels, spinal hyperexcitability, descending
facilitation, and aberrant sympathetic-somatic nervous-system interactions.
2
Neuropathic pain can be
debilitating, crippling, and even fatal for some patients. Treatments for
neuropathic pain commonly include nonpharmacological therapies, medications,
and invasive procedures such as spinal-cord stimulation.2
Conventional pain medications may mask the symptoms associated with
neuropathy; however, they are sometimes associated with significant side
effects and addiction profiles. Many patients have moderate to severe pain and
may require chronic use of multiple medications, which may lead to undesirable
adverse reactions and, ultimately, poor patient compliance. Pain is one of the
primary reasons patients seek medical attention. If it is improperly
controlled, quality of life can be affected, resulting in decreased
productivity at work, sleep deprivation, or anxiety or depression.3
The ReBuilder
The ReBuilder is a
noninvasive hand-held device approved by the FDA for the treatment of pain
(see Figure 1).4 This treatment device was designed based on
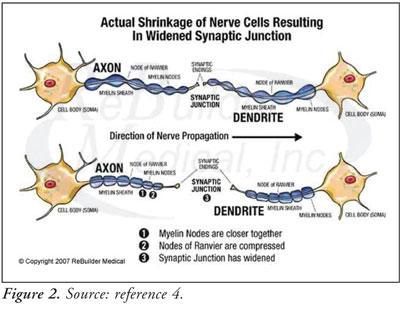
the premise that oxygen deficiency is responsible for physical atrophy of
nerve cells, which leads to the enlargement of the synaptic junction between
the axon of one cell and the dendrites of the next. As a result, it is more
difficult for normal-intensity electrical impulses to jump across this
synaptic gap, ultimately causing neuropathy (see Figure 2).4
The ReBuilder is designed to circumvent this gap by waking up dormant nerve
cells, relaxing shrinking nerve cells, and restoring normal plus/minus
polarity along the nerve axons and dendrites.
The ReBuilder works simultaneously
on three separate levels: stimulation of the nerves, stimulation of the
muscles, and combined electrostimulation. The first signal is designed to
stimulate the nerves by sending an electrical impulse with a very narrow
wave-form and a relatively high transient voltage: 40 to 90 volts AC. This
signal restores the nerve function and repolarizes synaptic junctions. The
second signal stimulates the muscles by a different, wider waveform with a
larger subthreshold amount of current under the curve and a much smaller
voltage (5 to 20 AC).4 Simultaneously stimulating the muscles of
the feet, calves, thighs, and buttocks, the ReBuilder evokes complete
relaxation between each contraction stimulus. This increases the flow of
oxygen-rich blood to the synaptic junctions, affording effective and efficient
conduction of nerve signals. Combined electro stimulation uses twin
electrical signals to stimulate the nerves and muscle cells. The manufacturer
states that the twin electrical signals cause the brain to release endorphins,
inducing a sense of well-being and reducing anxiety as well as physical and
emotional trauma. Reduction of pain will lead to improvement in patient
compliance and quality of life. Additionally, the ReBuilder features a
simultaneous weighted DC signal designed to stabilize the trigger threshold
that regulates the sensitivity of the nerve cell.4 By sending this
constant DC signal, the resting potential is held at a fixed voltage long
enough for the cell to stabilize itself and regain control.4

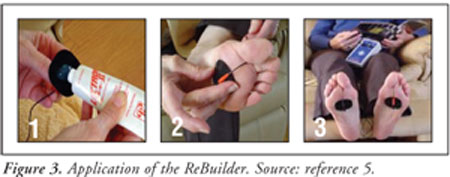
The ReBuilder is a
patient-friendly device that can be used to treat neuropathic pain. It
requires a 30-minute treatment window in which the patient applies conductive
gel to each of the signal pads. The pads are then placed on the soles of the
feet, the palms of the hands, the lower back, or the shoulders, as needed (see
Figure 3).5 Pain relief may last for four to six hours after
treatment.
Two models are available: the
3000 (personal) and the 2406 (clinical). Both contain the electric stimulator;
two lead sets and six adhesive signal pads; a twin-compartment footbath;
Cooling Cream; Electrolyte; Epsom salts; and two nonadhesive pads for use with
the footbath. The manufacturer states that the footbath improves patient
compliance because it delivers the signal across the entire surface of the
foot for comfort, causing vaso dila t- a tion within capillaries and
increased infusion of freshly oxygenated blood.4 Other application
methods are available, such as heated massage boots and conductive socks and
gloves. The heated boot vibrates and enables foot elevation.4
The 3000 personal model is
used for moderate neuropathy and has two separate outputs, affording the
patient the opportunity to concurrently treat two areas: the lower back and
the feet. Patients with chronic lower-back pain and muscle spasms may prefer
to use the 3000 model because of its portability and convenience. The 2406
clinical model is used for advanced neuropathy, especially neuropathy that
involves severe numbness. The 2406 model may be beneficial for visually
impaired or arthritic patients because of its large adjustment knobs and the
large printing on the case.
Efficacy
ReBuilder Medical,
Inc. conducted an internal feedback study evaluating 450 cases. This study
observed several different factors to determine the success rate in patients,
including average pain level, highest level of pain, and improvement in
quality of life. Initially and at the end of 10 treatment sessions, the
average pain level was calculated. Observations indicate an average reduction
in pain by 27.35% and an increase in quality of life by 81.14%. A decrease in
symptoms was reported by 95.76% of patients, and 74.56% of patients felt
immediate relief after the first session. Currently, several studies of the
ReBuilder are ongoing.6
Conclusion
Although several
pharmacologic modalities may be employed to address neuropathic pain, pain
control may not be achieved in all patients. The ReBuilder shows promise for
pain mitigation and increased overall quality of life. Additional information
about the device can be found at the ReBuilder Web site: www.rebuildermedical
.com/Is_it_easy_to_use.
References
1. The Neuropathy Association. Peripheral neuropathy: facts. Available at: www.neuropathy.org/ site/PageServer?pagename=About_
Facts. Accessed June 18, 2007.
2. Gilron I, Watson CP, Cahill CM, et al. Neuropathic pain: a practical guide for the clinician. Canadian Med Assoc J. 2006;176:265-275.
3. McCarberg BH, Billington R. Consequences of neuropathic pain: quality-of-life issues and associated cost. Am J Manage Care. 2006;12(suppl 9):S263-268.
4. ReBuilder Medical Technologies, Inc. Available at: www.rebuildermedical.com/. Accessed June 1, 2007.
5. SeekWellness. What is the ReBuilder? Available at: www.seek wellness.com/conditions/diabetes/rebuilder.htm. Accessed June 22, 2004.
6. ReBuilder Internal Studies.
Customer service department. Available at: www.rebuildermedical.com. Accessed
June 21, 2007.
To comment on this article, contact
editor@uspharmacist.com.





