US Pharm. 2010;35(11):HS2-HS8.
Surgeons face multiple bleeding challenges in the operating room. Operative bleeding challenges can contribute to postoperative complications and result in additional or prolonged procedures and even increased length of stay. Topical hemostats are used in the setting of surgery or trauma as adjuncts to maintain hemostasis. Topical hemostat products include gelatin sponges, collagens, fibrin sealants, and active thrombin preparations. These agents are applied locally to stop blood flow.1
Current health care has an emphasis on outcomes. With demand for improved technical procedures, the use of topical hemostats may help improve outcomes. There is often a knowledge gap for pharmacists with regard to topical hemostats. In some institutions, these products may be supplied to the operating room from the central supply and may not even be ordered or stocked by the pharmacy.2 Topical hemostats often get very little attention from the typical curriculum of today’s pharmacy schools. Moreover, pharmacists generally do not have a real connection with these products, as they are not in the surgical suite to see how hemostats are used in their institution. However, it is still important for pharmacists to be familiar with all formulary agents used within the institution.
Use of Hemostats
Topical hemostats are used intraoperatively foremost and postoperatively to a lesser extent.3 These agents can be used for diffuse raw surface bleeding, oozing venous type bleeds, bone bleeding, and needle-hole bleeding.4 Surgical bleeding adds a number of complications to the patient, such as altered vision in the surgical field, unstable hemodynamics, increased need for blood transfusions, increased operative time, increased risk of infection, increased cost, and increased overall mortality. Factors to consider when selecting a topical hemostat include the type of agent, the size of the wound, and the accessibility of the bleeding site. Other factors include the severity of the bleeding and the coagulation status of the patient. In addition, the medical and surgical history of the patient and the likelihood of reoperation must be taken into account when identifying bleeding risks and selecting appropriate topical hemostats.1 For localized small-vessel bleeding, thrombin, fibrin sealant with a surgical sponge, or thrombin with a sponge can be used. For more diffuse (venous) bleeding, the materials can be sprayed on.
The ideal topical hemostat would provide reliable and prompt control of bleeding. The desirable agent would have ease of storage (room temperature) and preparation (needle free); have immediate availability (stored in the surgical suite) and usability (for a variety of procedures); and ideally reduce the time spent in the operating room. In addition, the selected agent would demonstrate safety features and be affordable (by minimizing blood transfusions).1 In general, topical hemostats are classified into four categories: mechanical hemostats, active hemostats, flowable hemostats, and fibrin sealants (TABLE 1).1,5-7
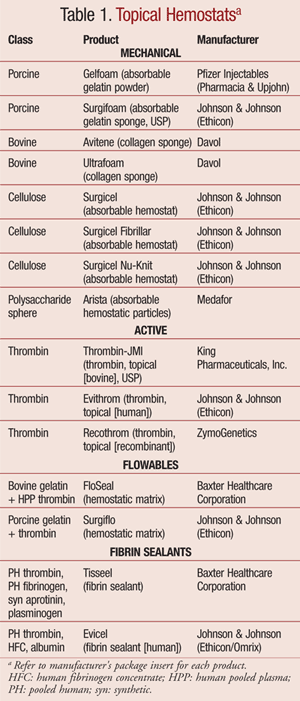
Mechanical Hemostats
Mechanical topical hemostat products are gelatin, collagen, cellulose, and polysaccharide based. Mechanical hemostats are applied as sponges and do not contain thrombin or any other active biologic compounds. They produce swelling and cause a mechanical barrier to bleeding and oozing. They can be used with saline or thrombin and are stored at room temperature. These products can produce abscess formation. Mechanical products are generally considered first-line agents because they are widely available in the operating room and are the least expensive topical hemostat.1
Examples of porcine gelatin products include Gelfoam (Pfizer) and Surgifoam (Johnson & Johnson, Ethicon). The Gelfoam products are used for all surgical procedures. Gelfoam Plus requires a needle for preparation and contains pooled human thrombin; thus, it carries a risk of viral transmission. The thrombin concentration in Gelfoam Plus is lower at 125 IU/mL compared to the other thrombin products, which are 800 to 1,200 IU/mL. Surgifoam is used for all surgical procedures but is not indicated for ophthalmic procedures.1
Bovine collagen products include Avitene (Davol) and Ultrafoam (Davol). These products can be used in all surgical procedures. Thrombin is not required to be used with Ultrafoam sponges. Avitene is available as flour and sheets. The flour may stick to gloves and surgical instruments. Surgicel, Surgicel Fibrillar, and Surgicel Nu-Knit (all Johnson & Johnson) are examples of oxidized cellulose products. The hemostatic effect of Surgicel is not enhanced with the addition of thrombin, as thrombin is destroyed by the low pH of the product. This line of products can be used for all surgical procedures but may create a dark gelatinous mass that may obscure the visual field during surgery.1
Arista (Medafor) is a plant-derived polysaccharide starch that acts like a sieve to dehydrate blood. This polysaccharide sphere concentrates the solid components of blood to help the blood-clotting proteins. These spheres are composed of sugar, and not more than 50 g should be used in patients with diabetes to avoid glucose overload. Studies have shown this product to be efficacious in reducing capillary, venous, and arteriole bleeding that cannot be treated by other conventional means. However, specific safety data have not been demonstrated in neurologic, urologic, and ophthalmic surgeries, and the product is not recommended for use with bone adhesives.1
Active Hemostats
Thrombin is a proteolytic enzyme that converts fibrinogen to fibrin. Topical thrombin has a direct clotting effect on exposed blood. The active hemostats include topical (bovine) thrombin (Thrombin-JMI), topical (human) thrombin (Evithrom), and topical (recombinant) thrombin (Recothrom). These agents are indicated as an aid to hemostasis whenever ongoing oozing blood and minor bleeding from capillaries and small venules are accessible. The active agents stimulate activity in the coagulation cascade and promote the conversion of fibrinogen to fibrin (FIGURE 1).8
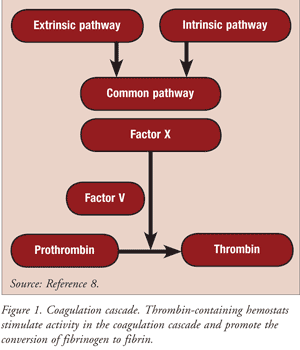
The first clinical use of topical bovine thrombin dates back to more than 60 years ago. In the 1970s, FDA approval of thrombin was grandfathered in. The use of topical thrombin has been described for over 100 uses, including spinal, neurologic, general, orthopedic, cardiac, thoracic, vascular, gynecologic, and dental procedures.5,9
Thrombin-JMI (King Pharmaceuticals) was approved in 1995.10 It is estimated that between 500,000 and 1 million patients have exposure to bovine thrombin on an annual basis in the U.S. With a broad indication for use, bovine thrombin has two vial sizes and can be sprayed on or applied with a sponge. In 1996, a black box warning was added to all bovine thrombin preparations. This warning notes occasional association with bovine thrombin and coagulopathies. These coagulopathies can range from minor laboratory abnormalities (altered prothrombin time [PT], partial thromboplastin time [PTT]) to minor bleeding (epistaxis or hematoma) to serious bleeding (persistent, uncontrolled, and life-threatening). This reaction is thought to be related to antibodies against bovine thrombin and/or factor V that cross-react with human coagulation factors.11 Factor V is an essential cofactor in the conversion of prothrombin to thrombin. Factor V inhibitors can cause inactivation or depletion of factor V. The true incidence of coagulopathy associated with bovine thrombin is unknown with either initial exposure or re-exposure. However, the risk of antibody formation increases with re-exposure to bovine thrombin.
The clinical features of immune-mediated coagulopathy (IMC) are quite variable and may be masked by other disease states. Antigen exposure (previous bovine thrombin administration) may not be well documented in the medical record.12 The reported time of clinical presentation of IMC is quite variable and may occur within 10 days with a mean of 32 days to months after exposure. Over 60 cases with known or presumed surgical exposure to bovine thrombin have been published. Bleeding was noted in approximately one half of these cases. Currently, there is a low clinical awareness for IMC.5,13-15 Although the newly formulated bovine-derived Thrombin-JMI contains significantly reduced levels of contaminants, coagulopathies can still occur.10,16
Hemorrhagic IMC is a diagnostic challenge. The clinical presentation may be quite variable and masked by other conditions such as anticoagulant or antiplatelet therapy, vitamin K or liver disease, disseminated intravascular coagulation, acidosis and hypothermia, and hemodilution due to blood loss.17,18 The management of hemorrhagic IMC often requires a hematology consult. Supportive care with extended follow-up of laboratory tests can manage a patient in the absence of overt bleeding. For the bleeding patient, packed red blood cells, platelet transfusions and immunosuppressive therapy (corticosteroids, cyclosporine), chemotherapy (cyclophosphamide, vincristine), or epsilon aminocaproic acid may be required.12,19-21
In 2007, the FDA approved a pooled human plasma thrombin source (Evithrom [Johnson & Johnson]). The final product undergoes viral inactivation treatment to ensure the safety of the product. This product has no black box warning but does carry a risk of viral transmission. Evithrom is stored frozen and must be unthawed prior to administration. Once reconstituted, the product can be stored in the refrigerator for 30 days and at room temperature for 24 hours.22
Recombinant topical thrombin (Recothrom [ZymoGenetics]) was FDA approved in 2008. This product is produced utilizing recombinant DNA and has comparable efficacy compared to bovine thrombin but is free from bovine or human plasma. It is produced via Chinese hamster ovary (CHO) host cell protein.23 Examples of other products produced via recombinant technology include human insulin, growth hormone, etanercept (Enbrel), bevacizumab (Avastin), and rituximab (Rituxan).24-26
Flowable Hemostats
Flowable hemostats have a thick but flowable consistency and contain a bovine or porcine gelatin matrix. Topical thrombin may be added. They are helpful when application is needed into difficult-to-reach surfaces or in a wet field. The products can be used for all surgical procedures other than ophthalmic. Surgiflo (Johnson & Johnson) contains porcine gelatin and can be mixed with thrombin.27 FloSeal (Baxter) contains bovine gelatin matrix and human pooled plasma thrombin.28
Fibrin Sealants
Fibrin sealants contain thrombin and fibrinogen, can be sprayed, and are helpful when a hemostat and sealant is required. Tisseel (Baxter) contains pooled human thrombin, pooled human fibrinogen, synthetic aprotinin, and plasminogen.29 The human thrombin carries a risk of viral transmission, and the product has a lengthy preparation. In 1998, Tisseel was the first surgical sealant approved in the U.S. Evicel (Johnson & Johnson) contains pooled human thrombin, human fibrinogen concentrate, and albumin. There is also the risk of viral transmission. This product has frozen storage and requires thawing.30
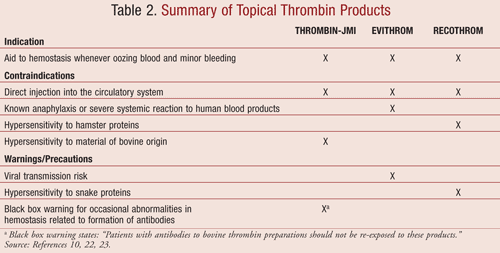
Topical Thrombin Products
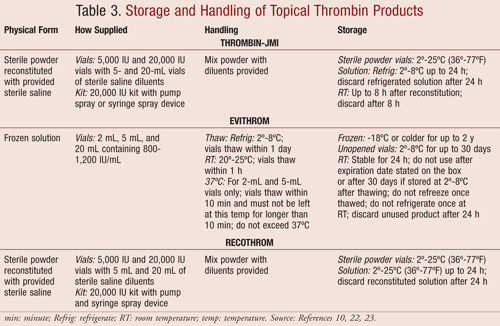
Bovine thrombin may be used alone or in combination with sponges, flowables, and fibrin sealants. Physicians, perfusionists, or nurses may not be familiar with the ingredients of topical hemostats. Physicians considering the use of topical bovine thrombin often do not know whether the patient has had previous exposure. There is no diagnostic test available to test for bovine thrombin antibodies. The best strategy is to limit the risk of IMC. Within the last few years, new topical thrombins with low rates of immunogenicity have been approved.15,31 TABLES 2 and 3 list a summary of the topical thrombin products and their storage and handling requirements.
Role of the Pharmacist
Pharmacists need to realize that perioperative bleeding may be associated with major clinical and economic consequences. Effective blood management programs involve minimization of blood products and optimal use of topical hemostats. Multiple topical hemostats are available, and each product may have different advantages and benefits. Bovine thrombin–associated coagulopathy is a potentially serious and unrecognized IMC, even though the incidence is unknown. A pharmacist in the health care setting is positioned to be involved with the development and monitoring of the multidisciplinary hemostatic formulary.21
Ideally, pharmacists and physicians, working together under the principles of the Pharmacy and Therapeutics (P&T) Committee, promote the safe and effective use of all formulary agents. Special considerations may be given to those agents with black box warnings. In addition, certain high-risk agents may be restricted to a specific level of patient care or require specialized protocols for administration. Finally, special training or credentialing may be required for some agents. Pharmacists should be well acquainted with all formulary agents used within their institution, and topical hemostats may be a medication category that pharmacists need to become more familiar with. The Joint Commission highlighted the importance of recognizing bovine thrombin–associated IMC and the threat it could pose to patient safety, not to mention the potential cost of treating this medical condition.32
All medications fall under the Joint Commission’s requirements for medication use. Medication products are to be stored, handled, and processed within institution-specific policies following formulary review. The Joint Commission’s National Patient Safety Goals indicate that all medications be accurately and completely reconciled across the continuum of care, which implies that the use of all medications is to be documented on the patient’s chart. The oversight of all medications used within the institution, including topical hemostats, typically falls under the auspices of the pharmacy staff.33,34 Topical hemostats are important adjunctive therapy to maintain hemostatis in surgical patients. With the knowledge of hemostasis and the coagulation cascade, pharmacists can interact with other health professionals and maximize the benefits of hemostatic agents.
REFERENCES
1. Spotnitz WD, Burks S. Hemostats, sealants, and adhesives: components of the surgical toolbox. Transfusion. 2008;48:1502-1516.
2. Lomax C, Traub O. Topical thrombins: benefits and risks. Pharmacotherapy. 2009;29:8S-12S.
3. Tomizawa Y. Clinical benefits and risk analysis of topical hemostats: a review. J Artif Organs.
4. Diesen DL, Lawson JH. Bovine thrombin: history, use and risk in the surgical patient. Vascular.
5. Kessler CM, Ortel TL. Recent developments in topical thrombins. Thromb Haemost. 2009;101:15-24.
6. Boucher BA, Traub O. Achieving hemostasis in the surgical field. Pharmacotherapy. 2009;29:2S-7S.
7. de la Torre RA, Bachman SL, Wheeler AA, et al. Hemostasis and hemostatic agents in minimally invasive surgery. Surgery. 2007;142(supp1 4):S39-S45.
8. Sarfati MR, DiLorenzo DJ, Kraiss LW, Galt SW. Severe coagulopathy following intraoperative use of topical thrombin. Ann Vasc Surg. 2004;18:349-351.
9. Cheng CM, Meyer-Massetti C, Kayser SR. A review of three stand-alone topical thrombins for surgical hemostasis. Clin Ther. 2009;31:32-41.
10. Thrombin-JMI (thrombin, topical [bovine], USP) package insert. Bristol, TN: King Pharmaceuticals Inc; November 2007.
11. Naoum JJ. Immune-mediated coagulopathy: a case report. Pharmacotherapy. 2009;29:13S-17S.
12. Ofosu FA, Crean S, Reynolds MW. A safety review of topical bovine thrombin-induced generation of antibodies to bovine proteins. Clin Ther. 2009;31:679-691.
13. Ness P, Creer M, Rodgers GM, et al. Building an immune-mediated coagulopathy consensus: early recognition and evaluation to enhance post-surgical patient safety. Patient Saf Surg. 2009;3:8.
14. Dunn BL, Uber WE, Ikonomidis JS. Topical thrombin preparations and their use in cardiac surgery. Open Access Surg. 2009;2:15-34.
15. Chapman WC, Singla N, Genyk Y, et al. A phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis. J Am Coll Surg. 2007;205:256-265.
16. Lawson JH, Lynn KA, Vanmatre RM. Antihuman factor V antibodies after use of relatively pure bovine thrombin. Ann Thorax Surg. 2005;79:1037-1038.
17. Dagi TF. The management of postoperative bleeding. Surg Clin North America. 2005;85:1191-1213.
18. Zimmerman LH. Causes and consequences of critical bleeding and mechanism of blood coagulation. Pharmacotherapy. 2007;27:45S-56S.
19. Streiff MB, Ness PM. Acquired FV inhibitors: a needleless iatrogenic complication of bovine thrombin exposure. Transfusion. 2002;42:18-26.
20. Franchini M, Veneri D. Acquired coagulation inhibitor-associated bleeding disorders: an update. Hematology. 2005;10:443-449.
21. Voils SA. Thrombin products: economic impact of immune-mediated coagulopathies and practical formulary considerations. Pharmacotherapy. 2009;29:18S-22S.
22. Evithrom (thrombin, topical [human]) package insert. Somerville, NJ: J&J Wound Management (Ethicon Inc); January 2009.
23. Recothrom (thrombin, topical [recombinant]) package insert. Seattle, WA: ZymoGenetics, Inc; January 2010.
24. Enbrel (etanercept) package insert. Thousand Oaks, CA: Immunex Corporation; November 2009.
25. Avastin (bevacizumab) package insert. South San Francisco, CA: Genentech Inc; July 2009.
26. Rituxan (rituximab) package insert. South San Francisco, CA: Genentech Inc; October 2009.
27. Surgiflo hemostatic matrix package insert. New Brunswick, NJ: Johnson & Johnson Medical Ltd; March 2007.
28. FloSeal hemostatic matrix package insert. Fremont, CA: Baxter Healthcare Corporation; March 2005.
29. Tisseel Fibrin Sealant package insert. Westlake Village, CA: Baxter Healthcare Corporation: January 2010.
30. Evicel fibrin sealant (human) package insert. Somerville, NJ: J&J Wound Management (Ethicon Inc/Omrix Biopharmaceuticals Ltd); November 2009.
31. Doria C, Fischer CP, Wood CG, et al. Phase 3, randomized, double-blind study of plasma-derived human thrombin verses bovine thrombin in achieving hemostasis in patients undergoing surgery. Curr Med Res Opin. 2008;24:785-794.
32. The Joint Commission. Bovine thrombin-associated IMC: a threat to patient safety and the bottom line. Joint Commission Perspect Patient Saf. 2010;10:5-6.
33. The Joint Commission. 2010 Accreditation Process Guide for Hospitals. Chicago, IL: JCAHO; 2010.
34. 2010 National Patient Safety Goals (NPSGs). Effective July 1, 2010. The Joint Commission. www.jointcommission.org/ 2005;8:137-142. 2008;16(suppl 1):S29-S36.
To comment on this article, contact rdavidson@uspharmacist.com.






