US Pharm. 2021;46(10):42-46.
ABSTRACT: About one in eight women will be diagnosed with breast cancer in her lifetime. Of these, 6% will have metastatic disease that carries a 5-year survival rate of about 29%. The year 2020 brought about FDA approval for three new agents targeting varying forms of metastatic breast cancer in the hope that more women will be able to successfully combat this stage of disease. The approval of Trodelvy provides a second-line treatment option for patients with triple-negative metastatic breast cancer. For patients with HER2+ metastatic breast cancer, Tukysa and Margenza are new second- and third-line options available.
In 2020, female breast cancer (FBC) was the most prevalent cancer worldwide and the fourth leading cause of cancer-related death.1 The incidence of FBC in the United States has remained constant over the past 15 years.2 It is estimated to affect 12.9% of women in their lifetime; about 281,550 new cases will be diagnosed in 2021. The average age at diagnosis is 63 years, with most diagnoses occurring between ages 55 and 74 years. For this reason, the U.S. Preventive Services Task Force recommends a screening mammography every 2 years for women with average risk who are aged 50 to 74 years old.3 This differs from the screening recommendations provided by the American Cancer Society, which suggests annual mammograms for women aged 45 to 54 years and then a transition to biennial screening for women aged 55 years and older.
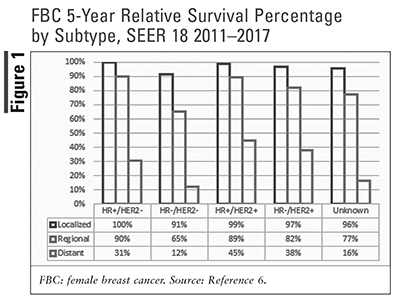
Screening mammograms are a method for early detection of breast cancer prior to a sign of disease, such as a palpable lump on a self or clinical breast exam. Early detection correlates to localized disease at diagnosis, which has an increased survival rate. Although there has been a steady decline in deaths related to FBC, those diagnosed with distant (metastatic) breast cancer still face a high likelihood of death. Metastatic breast cancer (MBC), also referred to as stage IV or advanced breast cancer, accounts for 6% of all breast cancer diagnoses. Breast cancer metastasis, or progression of cancer to distant organs, most commonly affects the lungs, liver, bone, and brain.4,5 The 5-year relative survival rate ranges from 12% to 45% (FIGURE 1).6 To combat this, research has been targeting metastatic cancer with three drugs receiving approval in 2020-2021: sacituzumab govitecan-hziy (Trodelvy), tucatinib (Tukysa), and margetuximab-cmkb (Margenza).7
To date, there is no single standard of care for MBC. Treatment goals for MBC consist of prolonging survival, palliating symptoms, and delaying the progression of the disease, since MBC remains incurable. Treatment for MBC includes chemotherapy (most common), surgery, radiation, hormonal therapy, immunotherapy, and gene therapy.6,8 Treatment plans are individualized based on the benefits and risk factors of therapeutic agents, previous treatment, and clinical status.
TRIPLE-NEGATIVE MBC
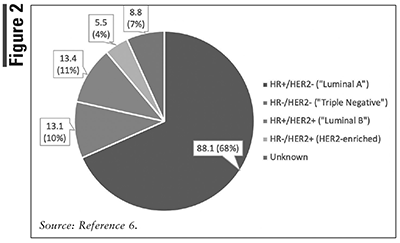
Triple-negative breast cancer (TNBC) is a form of breast cancer that lacks the presence of estrogen and progesterone receptors along with HER2 gene amplification.9 About 10% of breast cancers are TNBC (FIGURE 2).6 Unlike other types of breast cancer that typically occur in middle-aged and older women, TNBC is more likely to affect those younger than age 50 years.10 As routine screening in this population is not recommended and owing to the highly aggressive nature of TNBC, women are often diagnosed later, when progression of the disease has already occurred. In nonmetastatic disease, some of the highest pathologic complete response rates to neoadjuvant chemotherapy are seen. However, high relapse rates and distant metastasis, commonly to the brain and lungs, have triggered increased research and drug development into TNBC.
NEW DRUGS FOR mTNBC
Sacituzumab Govitecan-hziy (Trodelvy)
Sacituzumab govitecan-hziy received accelerated approval by the FDA in April 2020 for patients with metastatic triple-negative breast cancer (mTNBC) who have received at least two prior treatments with at least one for metastatic disease.11,12 Regular approval was granted in April 2021 for patients with unresectable locally advanced or mTNBC who have received two or more prior systemic therapies, at least one of which was for metastatic disease.13
Sacituzumab govitecan-hziy, an antibody-drug conjugate, is a topoisomerase I inhibitor (SN-38) coupled to a humanized antitrophoblast cell-surface antigen 2 (Trop-2) monoclonal antibody.14 Trop-2 is a transmembrane calcium signal transducer that has been linked to increased tumor growth, enhanced proliferation, and cell migration. It is often overexpressed in most human solid epithelial cancers, e.g., breast cancer.15 Topoisomerases are enzymes that unwind coiled DNA to facilitate replication and transcription. The selective binding of the Trop-2 monoclonal antibody allows for selective delivery of SN-38 to tumor cells.
The initial phase I/II basket design, open-label, single-group, multicenter trial enrolled 108 patients with mTNBC who had received two prior treatments for mTNBC.16 The objective response rate (ORR) was 33.3% (36 of 108 patients), including complete response in three patients (2.8%). Duration of treatment with sacituzumab govitecan-hziy is twice the length of previous anticancer treatments (5.1 vs. 2.5 months, respectively). A randomized phase III, multicenter trial enrolled 468 patients with mTNBC without evidence of brain metastases who had relapsed or were refractory to two or more previous standard chemotherapy regimens (to include a taxane) for unresectable, locally advanced, or metastatic disease.14 Patients were randomly assigned in a 1:1 ratio to sacituzumab govitecan-hziy (235 patients) or single-agent chemotherapy (233 patients). The primary endpoint was progression-free survival (PFS) among patients without brain metastases. The median PFS withs sacituzumab govitecan-hziy was three times longer compared with chemotherapy (5.6 vs. 1.7 months respectively), and the medium overall survival (OS) was 12.1 versus 1.7 months. The ORR was 35% (82 of 235 patients without metastases) including complete response in 10 patients (4%).
The recommended dose of sacituzumab govitecan-hziy is 10 mg/kg IV infusion given on Days 1 and 8 of 21-day continuous treatment cycles until disease progression or unacceptable toxicity. All patients should be premedicated with antipyretics along with H1 and H2 blockers prior to infusion to prevent infusion-related reactions. Corticosteroids are recommended for patients with prior infusion-related reactions. Premedication for the prevention of chemotherapy-induced nausea and vomiting is also recommended. The black box warning (BBW) for this drug is severe neutropenia and diarrhea. When the absolute neutrophil count is below 1,500/mm3 or neutropenic fever occurs, sacituzumab govitecan-hziy should be withheld. There are no mild hepatic adjustments; adjustments for renal and severe hepatic conditions have not been established. Common side effects include fatigue, hair loss, anemia, constipation, decreased appetite, rash, and abdominal pain.12
Her2+ MBC
Human epidermal growth factor receptor 2 (HER2)-positive breast cancer accounted for 15% of all FBC cases from 2014-2018 and is associated with 5-year relative survival rates ranging from 38% to 99% (FIGURES 1 and 2).6 The HER2 receptor gene codes for the HER2 protein, which potentially promotes proliferation of cancerous cells.17 In normal body conditions, the HER2 gene is regulated by tumor-suppressor genes to keep a balance among replicating cells across the body. The HER2 family of genes is known as proto-oncogenes or a set of genes that, when activated, are uncontrollably and rapidly dividing; this rapid and uncontrolled division of cells may lead to cancer.18
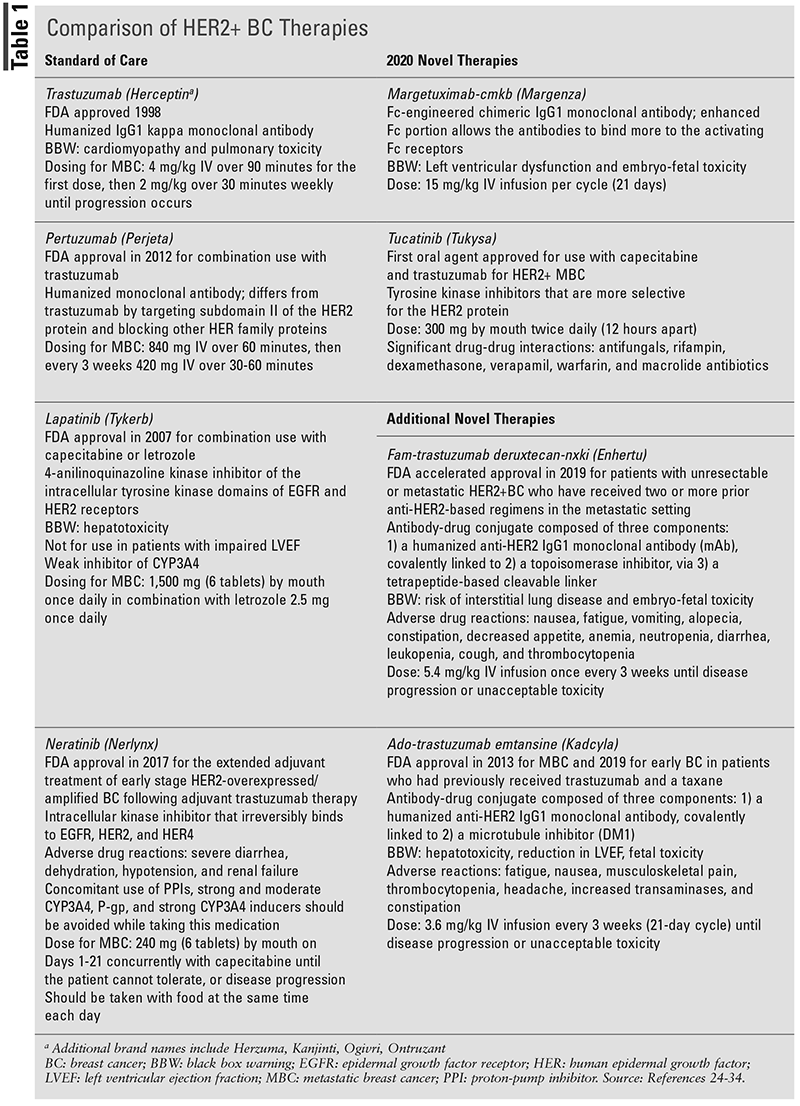
Due to emerging new therapies targeting the HER2 protein, screening with the immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH) test is recommended.18 Currently, for patients diagnosed with HER2+ breast cancer, the standard of care is chemotherapy plus trastuzumab with or without adjuvant endocrine therapy (TABLE 1). According to the 2021 National Comprehensive Cance Network guidelines, patients can receive various combinations of chemotherapy; however, all of the regimens include trastuzumab with or without pertuzumab to target the HER2+ cancerous cells.19
NEW DRUGS FOR HER2+ MBC
Margetuximab-cmkb (Margenza)
Margetuximab-cmkb, a chimeric Fc-engineered IgG1 monoclonal antibody derived from Chinese hamster ovary cells, received FDA approval for use in December 2020.20 This biologic works as a HER2/neu receptor antagonist indicated for patients with MBC who have already undergone two or more anti-HER2 therapies. Margetuximab-cmkb works by binding to the HER2 protein on tumor cells, inhibiting the proliferation of the cell, and inducing an immune-mediated antibody-dependent cellular cytotoxic reaction, which may induce an infusion-related reaction. During the SOPHIA trial, 536 patients were randomized to receive margetuximab-cmkb with chemotherapy versus trastuzumab with chemotherapy.21 The trial protocol allowed for the use of one of four systemic chemotherapy options, capecitabine, eribulin, gemcitabine, or vinorelbine, used in the salvage setting for advanced breast cancer. The population studied was predominately Caucasian, had a mean age of 55 years, and previously received one or two prior therapies (most commonly a taxane). Margetuximab-cmkb showed a 24% PFS relative risk reduction compared with trastuzumab therapy, thus meeting the primary endpoint. Median OS was 21.6 months with margetuximab-cmkb versus 19.8 months with trastuzumab (P = .33).
Margetuximab-cmkb is dosed at 15 mg/kg administered as an IV infusion. There are no renal dose adjustments necessary for patients with a creatinine clearance (CrCl) greater than 30 mL/min; however lower CrCl rates and end-stage renal disease have not yet been studied. For patients with mild or moderate hepatic impairment, there are no dose adjustments listed in the package insert. Left ventricular dysfunction and embryo-fetal toxicity are listed as BBWs. For this reason, patients should have an evaluation of left ventricular ejection fraction prior to and during therapy, and females should be screened for pregnancy. The use of anthracyclines is not recommended until 4 months post margetuximab-cmkb therapy, due to the increased risk of left ventricular dysfunction possible with both agents.6 Other common adverse drug reactions such as fatigue, nausea/vomiting, decreased appetite, abdominal pain, and pain in the extremities, are similar to other anticancer treatments.
Tucatinib (Tukysa)
Tucatinib, a tyrosine kinase inhibitor of the HER2 protein, exerts antitumor growth by inhibiting the phosphorylation of HER2 and HER3.22 Tucatinib, an oral anticancer agent given in combination with trastuzumab and capecitabine, is indicated for adult patients with metastatic HER2+ breast cancer, including those with brain metastases and unresectable tumors. Patients must have previously received one or more anti-HER2 regimens for metastatic cancer. The HER2CLIMB study, a double-blind, randomized trial, investigated tucatinib/trastuzumab/capecitabine versus placebo/trastuzumab/capecitabine.23 The PFS at 1 year was 33.1% for tucatinib and 12.3% for placebo (hazard ratio [HR] 0.54; 95% CI, 0.42-0.71; P <.001). At 2 years, the OS of patients was 44.9% for the tucatinib group and 26.6% for the placebo group (HR 0.66; 95% CI, 0.50-0.88; P = .005).
The recommended dose for tucatinib is 300 mg (2 tablets) by mouth twice daily in combination with trastuzumab and capecitabine until disease progression or unacceptable toxicity. Tablets should be swallowed whole, ideally 12 hours apart at a similar time each day, and can be taken without regard to meals. There are no renal adjustments listed for tucatinib, but because the combination of therapy uses capecitabine, patients with severe renal impairment have a contraindication and should not receive this therapy. In mild or moderate hepatic impairment (Child-Pugh class A or B), there are no necessary adjustments; however, patients with severe hepatic impairment (Child-Pugh class C) will require a dose reduction to 200 mg twice daily. The most common adverse reactions reported were diarrhea, hepatotoxicity, nausea, vomiting, anemia, headache, rash, and palmar-plantar erythrodysesthesia. Major warnings listed include diarrhea, hepatotoxicity, and embryo-fetal toxicity. Antidiarrheals are recommended based on severity, and liver-function tests should be monitored prior to and while continuing therapy. Pregnant patients or those trying to conceive should be counseled, as deformities in offspring were seen in the animal trials. Women who are lactating should be advised by practitioners to avoid breast-feeding during treatment and for at least 1 week after discontinuing treatment. Significant drug interactions include moderate CYP2C8 inducers, moderate or strong CYP2C8 inhibitors, strong CYP3A substrates, and P-gp substrates; concomitant use with these medications should be avoided.22
CONCLUSION
The year 2020 saw three novel therapies for MBC receive FDA approval. Despite these advances in treatment, early detection will always provide the best odds for survival. Pharmacists in all practice settings can help to educate women on the importance of completing screening mammograms. With continued drug development, survival rates for patients with MBC will likely continue to rise.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
2. National Institutes of Health; National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer stat facts: female breast cancer. https://seer.cancer.gov/statfacts/html/breast.html. Accessed July 31, 2021.
3. CDC. Breast cancer screening guidelines for women. www.cdc.gov/cancer/breast/pdf/breast-cancer-screening-guidelines-508.pdf. Accessed September 10, 2021.
4. Al-Mahmood S, Sapiezynski J, Garbuzenko OB, Minko T. Metastatic and triple-negative breast cancer: challenges and treatment options. Drug Deliv Transl Res. 2018;8(5):1483-1507.
5. National Cancer Institute. Cancer of the breast (female). Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/breast.html. Accessed July 2, 2021.
6. National Cancer Institute. Female breast cancer subtypes. Cancer Stat Facts. SEER. https://seer.cancer.gov/statfacts/html/breast-subtypes.html. Accessed June 29, 2021.
7. FDA. Novel drug approvals for 2020. www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2020. Accessed June 17, 2021.
8. O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist. 2005;10(S3):20-29.|
9. CDC. Triple-negative breast cancer. www.cdc.gov/cancer/breast/triple-negative.htm. Accessed September 10, 2021.
10. Adel NG. Current treatment landscape and emerging therapies for metastatic triple-negative breast cancer. Am J Manag Care. 2021;27(suppl 5):S87-S96.
11. Syed YY. Sacituzumab govitecan: first approval. Drugs. 2020;80(10):1019-1025.
12. Trodelvy—sacituzumab govitecan hzly package insert. Morris Plains, NJ: Immunomedics Inc; 2020.
13. FDA. FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer. www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-sacituzumab-govitecan-triple-negative-breast-cancer. Accessed July 25, 2021.
14. Bardia A, Hurvitz SA, Tolaney SM, et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384:1529-1541.
15. Goldenberg DM, Stein R, Sharkey RM. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget. 2018;9(48):28989-29006.
16. Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab govitecan-hziy in refractory metastatic triple-negative breast cancer. N Engl J Med. 2019;380(8):741-751.|
17. Giridhar K. HER2-positive breast cancer. What is it? Mayo Clinic. Published April 7, 2020. www.mayoclinic.org/breast-cancer/expert-answers/faq-20058066. Accessed June 29, 2021.
18. American Cancer Society. Oncogenes and tumor suppressor genes. www.cancer.org/cancer/cancer-causes/genetics/genes-and-cancer/oncogenes-tumor-suppressor-genes.html. Published June 25, 2014. Accessed June 29, 2021.
19. National Comprehensive Cancer Network. Breast cancer (version 5.2021). www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed June 30, 2021.
20. Margenza (margetuximab-cmkb) package insert. Rockville, MD: MacroGenics, Inc; 2021.
21. Rugo HS, Im SA, Cardoso F, et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: phase 3 randomized clinical trial. JAMA Oncol. 2021;7(4):573-584.
22. Tukysa (tucatinib) package insert. Bothell, Wasington: Seattle Genetics Inc; 2020.
23. Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-Positive Metastatic Breast Cancer [published correction appears in N Engl J Med. 2020 Feb 6;382(6):586]. N Engl J Med. 2020;382(7):597-609.
24. Trastuzumab (intravenous route) description and brand names. Mayo Clinic. July 1, 2021. www.mayoclinic.org/drugs-supplements/trastuzumab-intravenous-route/description/drg-20067130. Accessed July 31, 2021.
25. Pertuzumab (intravenous route) description and brand names. Mayo Clinic. February 1, 2021. www.mayoclinic.org/drugs-supplements/pertuzumab-intravenous-route/description/drg-20075621. Accessed July 31, 2021.
26. Herceptin: Biotechnology breakthrough in breast cancer wins FDA approval. Drugs.com. www.drugs.com/newdrugs/herceptin-biotechnology-breakthrough-breast-cancer-wins-fda-approval-4878.html. Accessed July 31, 2021.
27. Herceptin (trastuzumab) package insert. South San Francisco, CA: Genentech, Inc. Published September 26, 2003. www.accessdata.fda.gov/drugsatfda_docs/label/2000/trasgen020900lb.htm. Accessed July 31, 2021.
28. Margenza (margetuximab-cmkb) package insert. Rockville, MD: MacroGenics, Inc; 2021.
29. Perjeta (pertuzumab) package insert. South San Francisco, CA:Genentech Inc; 2021.
30. How perjeta (pertuzumab) is designed to work. www.perjeta.com/hcp/how-perjeta-works.html. Accessed July 31, 2021.
31. Tykerb (lapatinib) package insert. Research Triangle Park, NC: GlaxoSmithKline; 2018.
32. Nerlynx (neratinib) package insert. Los Angeles, CA: Buma Biotechnology, Inc. 2017.|
33. FDA. FDA approves fam-trastuzumab deruxtecan-nxki for unresectable or metastatic HER2-positive breast cancer. www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-unresectable-or-metastatic-her2-positive-breast-cancer. Accessed August 1, 2021.
34. FDA. Kadcyla (ado-trastuzumab emtansine) package insert. South San Francisco, CA: Genentech, Inc; 2013.
To comment on this article, contact rdavidson@uspharcist.com.






