US Pharm. 2023;48(10):14.
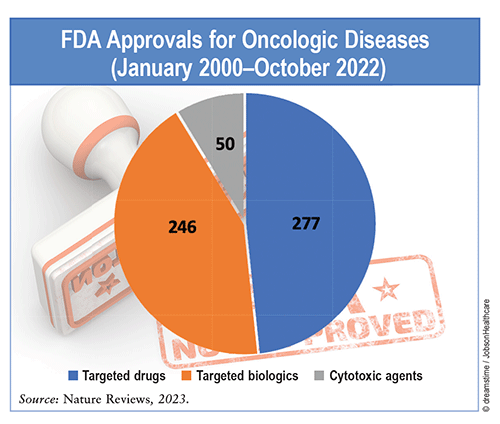
Over the past 30 years, more than 300 drugs and biologics have received accelerated approval from the FDA’s Center for Drug Evaluation and Research. Nearly 35% of these approvals have been for oncologic diseases, reflecting the changing landscape in new and novel therapeutic approaches for cancer. From January 2000 to October 2022, a total of 573 agents were approved for various oncologic indications, including targeted drugs (48%), biological therapies (43%), and cytotoxic treatments (9%). Seventy-one percent of agents underwent the regular approval process, and 29% received accelerated approval. The FDA has approved 25 new oncology therapeutics since January 2023.
Disease Sites: In the United States, age-adjusted mortality from all cancer sites has declined, from 206/100,000 for 1995 through 1999 to 155.5/100,000 for 2014 through 2018. Since 2014, therapeutic approvals have increased for breast cancer, leukemia, and lymphoma. This upward trend has also been detected for skin and thyroid cancers after an absence of therapeutic approvals between 2000 and 2010. Most recently, the majority of FDA approvals have been for lung cancers, lymphoma, genitourinary cancers, breast cancer, and leukemia (each receiving ≥35 approved products in the previous 8 years).
Product Classes: Therapeutics for oncologic diseases are classified into three product groups: targeted drugs, targeted biologics, and cytotoxic agents. These groups comprise 31 different classes based on mechanisms of action. Kinase inhibitors (targeted drugs) had the highest proportion of approvals (31%) among all classes between 2000 and 2021. In 2022, kinase inhibitors had the same proportion of approvals as immune checkpoint inhibitors (targeted biologics), which had the second most approvals (19%) in the years prior despite only entering the market in 2011. Antitumor-associated antigen antibodies (targeted biologics) had the third most approvals (10%). Approvals of cytotoxic drug classes (e.g., DNA-damaging agents, antimetabolites, and microtubule inhibitors) had the least approvals of the product groups, with most approvals occurring before 2010; however, the number of approved indications for targeted drugs or biologics in combination with cytotoxic drugs has increased since 2010.
Biomarker Use: Biomarkers used to identify certain cancers and cancer subtypes are increasingly guiding drug development and the identification of patients most likely to benefit from treatment. Biomarkers were used in 32% of approvals between 2000 and 2004, 30% between 2005 and 2009, and 43% between 2020 and 2022. From 2000 to 2022, biomarkers were most commonly used in approvals for targeted drugs (49%), followed by targeted biologics (35%). In the same time period, 61.1% of oncologic products were approved for use in biomarker-unselected populations and 38.9% approved for biomarker-defined populations. Innovations and advancements in therapeutic approaches for cancer are promising and will hopefully lead to improvements in patient outcomes.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






