US Pharm. 2017;42(1):41-44.
ABSTRACT: Trigeminal neuralgia (TGN) is a sudden onset, short-duration, yet debilitating neuropathic pain arising from the compression of the fifth cranial nerve, precipitated by daily activities such as chewing and speaking. This chronic condition is most common in older females, affecting up to 27 per 100,000 individuals worldwide. The first-line pharmacologic treatment for TGN is the anticonvulsant carbamazepine, with oxcarbazepine utilized for its similar mechanism but milder adverse-effect profile. Second-line drug therapies (baclofen, lamotrigine) are considered useful adjuncts, with less-well-studied medications or surgery reserved if standard treatment alone is ineffective or not tolerated by the patient.
Trigeminal neuralgia (TGN), or tic douloureux, is a rapid onset of stabbing, unilateral facial pain, lasting seconds to minutes, triggered by simple activities such as eating, brushing teeth, talking, or being exposed to a burst of cold air. TGN is estimated to affect approximately 12.6 to 27 per 100,000 individuals worldwide1,2 and is most common in females over the age of 50 years.3 In the United States, the prevalence of TGN is 15.5 cases per 100,000.4
This “lightning bolt” of pain originates from the fifth cranial nerve, which has three divisions providing sensation to various facial areas. The ophthalmic nerve (V1) sustains feeling to the eyes and forehead, the maxillary nerve (V2) to the cheek and upper lip region, while the mandibular nerve (V3) innervates the jaw area, which is involved in biting, chewing, and swallowing. TGN pain originates from the maxillary and mandibular divisions. TGN is associated with higher rates of depression, since the patient’s quality of life can be affected by chronic pain, and antidepressants can be necessary.5
Pathogenesis and Diagnosis
The mechanisms involved in TGN pathogenesis are not clearly understood. It is thought that vascular compression, typically venous or arterial loops at the trigeminal nerve entry into the pons, results in focal trigeminal nerve demyelination.6-8 TGN is categorized in three ways: idiopathic has no clear cause, classical is caused by compression of cranial nerve V, and secondary is a result of an underlying disease, such as a brain tumor or multiple sclerosis.9 Clinical findings, history, and a detailed examination of the head, neck, teeth, and jaw remain the primary methods to exclude other causes of neuropathic pain. Neuroimaging by an MRI is used diagnostically when nerve compression is suspected or if surgery should be considered. Drug therapy remains the primary treatment for TGN.
First-Line Drug Therapy
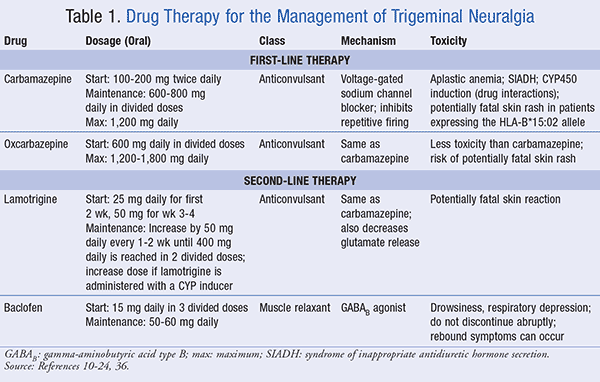
Carbamazepine: Based on clinical studies, carbamazepine is the estab-lished first-line treatment option for TGN; it is also the only FDA-approved drug with a specific indication for TGN.10-13 The usual starting dose of carbamazepine is 100 to 200 mg twice daily, administered orally, which can be increased as tolerated to a maintenance dose of 600 to 800 mg daily in divided doses. The maximum dose should not exceed 1,200 mg daily. These slow titrations in dosage reduce central nervous system (CNS) effects, such as drowsiness, dizziness, ataxia, and nystagmus. Carbamazepine is a voltage-gated sodium channel blocker, which stops the propagation of the action potential, the mechanism for treating epilepsy. Inhibition of this “repetitive firing” makes carbamazepine the appropriate primary treatment for TGN. Carbamazepine is also indicated for the treatment of patients with bipolar disease who are unable to tolerate other drugs for mania, such as lithium or valproate.10-13
Carbamazepine can alter bone marrow status, leading to a reduction of red blood cells, white blood cells, and platelets, a condition known as aplastic anemia. Thus, patients should be counseled to know the signs and symptoms of myelosuppression, with routine CBCs performed during the first 3 months of treatment, and the frequency being determined by the results.14 Carbamazepine stimulates the release of antidiuretic hormone (ADH), enhancing water reabsorption and the likelihood of hyponatremia, which can have a negative impact in older patients treated for TGN. This population can also experience changes in bone mineralization, as a decrease in vitamin D levels has been observed to occur with chronic carbamazepine administration.15
Carbamazepine is a well-known inducer of CYP450 3A4, with significant drug interactions reported, such as a decreased warfarin or oral contraceptive efficacy.16 Higher levels of serum carbamazepine result if the drug is concurrently administered with inhibitors of CYP450, including macrolide antibiotics, valproate, azole antifungals, and grapefruit juice. These effects taken together can be challenging to the physician treating an elderly patient, where the incidence of TGN is the highest.17
Carbamazepine is contraindicated in persons of east and southeast Asian populations who have tested positive for the HLA-B*15:02 allele.18 These individuals are at an increased risk for the development of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN). While this reaction is highly unlikely, it is fatal, and carbamazepine should be promptly discontinued if a rash is reported.
Oxcarbazepine: Oxcarbazepine is the keto analogue of carbamazepine, a prodrug converted to an active metabolite, a 10-monohydroxy derivative. The drug is started as an oral dose of 600 mg daily and increased by 300 mg to a total daily dose of 1,200 to 1,800 mg daily. The mechanism of action is the same as carbamazepine, but oxcarbazepine also reduces the activity of high-voltage activated calcium channels. Oxcarbazepine has less adverse effects and drug interactions than carbamazepine, and it is a suitable alternative for patients of advanced age with kidney, liver, and cardiac dysfunction. Since oxcarbazepine is an analogue of carbamazepine, it should also be avoided in patients expressing the genetic HLA-B*15:02 allele because of the rare skin reaction.19,20
In summary, carbamazepine and oxcarbazepine remain the gold standard for the pharmacotherapy of TGN. Anticonvulsants should be carefully titrated, with frequent monitoring for adverse effects and drug interactions. TABLE 1 summarizes carbamazepine and oxcarbazepine as first-line treatments for TGN.
Second-line Drug Therapy
The following drugs are reserved for patients who do not respond to carbamazepine and oxcarbazepine, or have a concern with adverse effects or drug interactions, as they are considered to be adjunctive for TGN management. In addition, there are fewer studies available for evidence of their efficacy.
Lamotrigine: Lamotrigine is an anticonvulsant, which suppresses the rapid firing of neurons and produces a voltage- and use-dependent blockade of sodium (Na+) channels, stabilizing neuronal membranes. There is also a decrease in glutamate release, the excitatory neurotransmitter. For TGN, lamotrigine provides an effective adjunctive therapy.21 Lamotrigine is administered orally in a 25 mg daily dose for the first 2 weeks, and then increased to 50 mg daily for weeks 3 and 4, until the total dose of lamotrigine reaches 400 mg per day (in two divided doses). Patients receiving concurrent therapy with inducers of CYP3A4 should be started with lamotrigine at a dose of 50 mg once daily, titrating upward as needed to 100 mg once daily at week 3, 200 mg once daily at week 5, 300 mg once daily at week 6, and 400 mg once daily at week 7. Patients taking an inhibitor of CYP3A4 should begin with a lower dose (12.5-25 mg) every other day, and be increased by 25 mg every 2 weeks, to a total of 400 mg per day. Potentially fatal skin reactions were reported with lamotrigine treatment, especially in the initial weeks of therapy, and patients should be instructed to promptly discontinue the drug at the first sign of rash.22
Baclofen: Baclofen is a skeletal muscle relaxant, acting as an agonist of gamma-aminobutyric acid type B (GABAB). Baclofen treatment allows for an increased chloride ion influx and closure of presynaptic calcium channels, which reduces the release of excitatory transmitters in the CNS to reduce muscle spasms. For pain management, baclofen reduces substance P in the spinal cord. The starting dose of baclofen for TGN is 15 mg daily administered orally in three divided doses, with gradual titration to a maintenance dose of 50 to 60 mg daily. For TGN, baclofen is observed to reduce the number of painful episodes and extend remission.23
By the potentiation of GABA, baclofen can induce drowsiness and respiratory depression in the event of an overdose. The drug should be slowly tapered and abrupt withdrawal is discouraged, as rebound spasticity, hallucinations, and seizures can occur.24
TABLE 1 summarizes the actions of lamotrigine and baclofen as second-line drug therapy for TGN.
Refractory Therapy
Gabapentin: Gabapentin is an analogue of GABA observed to be valuable for the treatment of neuropathic pain.25 The drug modulates the release of GABA, with no direct receptor action. In the CNS, gabapentin binds the N-type calcium channels, resulting in a decrease of calcium entry into the neurons. Gabapentin is started orally at a dose of 300 mg daily for TGN and can be increased by 300 mg every 2 to 3 days as tolerated. The maximum daily dose of gabapentin is 1,800 mg. The most common effects associated with gabapentin are drowsiness, dizziness, ataxia, headache, and tremor. There are minimal drug interactions associated with this GABA analogue.
Botulinum Toxin Type A (Botox-A): Botox-A has been observed to be an effective therapy for several neurologic pathologies, including headache.26-28 It prevents the calcium-dependent release of acetylcholine to induce muscle relaxation. Although the efficacy of this toxin in TGN is not totally understood, a release of nociceptive modulating peptides is thought to inhibit central and peripheral sensitization.29
In several clinical studies, the standard dose of Botox-A for the treatment of TGN was 25 to 100 U injected into the trigger zones, with pain relief lasting up to 12 weeks. Reported adverse effects included facial asymmetry and edema at the site of injection, which was tolerated by patients with a short recovery period.30-32 Further studies will be required to establish the long-term clinical outcome of Botox-A in TGN treatment.
Other Agents: Patients may experience breakthrough pain or an increased duration and intensity of TGN, which requires the use of potent opioids analgesics, such as morphine or oxycodone. The concern with the use of mu-opioid receptor agonists remains the potential for extreme sedation, addiction, and respiratory depression in the event of overdose.33
The dopamine blocker pimozide has been observed to have some benefit for TGN that is not responsive to first-line therapy with carbamazepine.34 However, there is a concern for extrapyramidal effects and cardiac arrhythmias with this dopamine2 (D2) antagonist, especially in older patients.34 Thus, pimozide is avoided by most clinicians for TGN therapy.
Lidocaine is a sodium channel blocker, which stops action potential propagation in the central and peripheral nervous systems. The pharmacokinetics of this anesthetic limits its use, as its short half-life reduces lidocaine duration of analgesia to no more than 24 hours, when administered via IV for TGN.35 In addition, higher doses of lidocaine can reduce myocardial conduction and induce tremors and abnormal sensations in the extremities.35
Fosphenytoin is a prodrug of the anticonvulsant phenytoin. Patients who have become refractory to oral medications, presenting in acute crisis of TGN, can experience pain relief of approximately 48 hours’ duration with this sodium channel blocker, until other pharmaco-therapeutic agents are considered or to provide analgesia before surgical intervention.36
Surgery: Surgery is reserved for patients who have tried at least three drugs without success or cannot tolerate the adverse effects or drug interactions. Surgery should be carefully considered, as certain procedures are invasive, utilize anesthesia, and increase the likelihood of infections. The most commonly used methods include microvascular decompression, rhizotomy, and gamma knife radiosurgery.37
Conclusion
Pharmacologic therapy remains the initial treatment for patients with TGN, with the anticonvulsant carbamazepine being the established first-line therapy. Second-line drugs are added if the patient is unable to tolerate the adverse effects or drug interactions. Pharmacists play a unique role in monitoring the patient for these outcomes. Clinical studies have expanded the possible role of other drugs for the treatment of this neuropathic pain, and these mechanisms should be further evaluated as options to manage this chronic pain in the future.
REFERENCES
1. Koopman JS, Dieleman JP, Huygen FJ, et al. Incidence of facial pain in the general population. Pain. 2009;147(1-3):122-127.
2. Hall GC, Carroll D, McQuay HJ. Primary care incidence and treatment of four neuropathic pain conditions: a descriptive study, 2002-2005. BMC Fam Pract. 2008;9:26.
3. Liu L, Wang H, Liu N, et al. Osteoporosis in the jawbones: a correlative factor of primary trigeminal neuralgia. Med Sci Monit. 2014;20:1481-1485.
4. Shaparin N, Gritsenko K, Fernandez Garcia-Roves D, et al. Peripheral neuromodulation for the treatment of refractory trigeminal neuralgia. Pain Res Manag. 2015;20(2):63-66.
5. Hals EKB, Stubhaug A. Mental and somatic co-morbidities in chronic orofacial pain conditions: pain patients in need of multiprofessional team approach. Scand J Pain. 2011;2(4):153-154.
6. Jannetta PJ. Arterial compression of the trigeminal nerve at the pons in patients with trigeminal neuralgia. J Neurosurg. 1967;26(1 suppl):159-162.
7. Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain. 2002;18:4-13.
8. Devor M, Govrin-Lippmann R, Rappaport ZH. Mechanism of trigeminal neuralgia: an ultrastructural analysis of trigeminal root specimens obtained during microvascular decompression surgery. J Neurosurg. 2002;96:532-543.
9. Cruccu G, Finnerup NB, Jensen TS, et al. Trigeminal neuralgia: new classification and diagnostic grading for practice and research. Neurology. 2016;87(2):220-228.
10. Gronseth G, Cruccu G, Alksne J, et al. Practice parameter: the diagnostic evaluation and treatment of trigeminal neuralgia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology and the European Federation of Neurological Societies. Neurology. 2008;71:1183-1190.
11. Campbell FG, Graham JG, Zilkha KJ. Clinical trial of carbazepine (Tegretol) in trigeminal neuralgia. J Neurol Neurosurg Psychiatry. 1966;29:265-267.
12. Rockliff BW, Davis EH. Controlled sequential trials of carbamazepine in trigeminal neuralgia. Arch Neurol. 1966;15:129-136.
13. Killian JM, Fromm GH. Carbamazepine in the treatment of neuralgia. Use of side effects. Arch Neurol. 1968;19:129-136.
14. Sobotka JL, Alexander B, Cook BL. A review of carbamazepine’s hema-tologic reactions and monitoring recommendations. DICP. 1990;24(12):1214-1219.
15. Mintzer S, Boppana P, Toguri J, et al. Vitamin D levels and bone turnover in epilepsy patients taking carbamazepine or oxcarbazepine. Epilepsia. 2006;47(3):510-515.
16. Perucca E. Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol. 2006;61(3):246-255.
17. Oomens MA, Forouzanfar T. Pharmaceutical management of trigeminal neuralgia in the elderly. Drugs Aging. 2015;32(9):717-726.
18. Locharernkul C, Shotelersuk V, Hirankarn N. Pharmacogenetic screening of carbamazepine-induced severe cutaneous allergic reactions. J Clin Neurosci. 2011;18(10):1289-1294.
19. Guleria VS, Sharda C, Rana T, Sood AK. Oxcarbazepine-induced toxic epidermal necrolysis—a rare case report. Indian J Pharmacol. 2015;47(4):459-461.
20. Lin LC, Lai PC, Yang SF, Yang RC. Oxcarbazepine-induced Stevens-Johnson syndrome: a case report. Kaohsiung J Med Sci. 2009;25(2):82-86.
21. Zakrzewska JM, Chaudhry Z, Nurmikko TJ, et al. Lamotrigine (Lamictal) in refractory trigeminal neuralgia: results from a double-blind placebo controlled crossover trial. Pain. 1997;73(2):223-230.22. Wang XQ, Lv B, Wang HF, et al. Lamotrigine-induced severe cutaneous adverse reaction: update data from 1999-2014. J Clin Neurosci. 2015;22(6):1005-1011.
23. Fromm GH, Terrence CF, Chattha AS. Baclofen in the treatment of trigeminal neuralgia: double-blind study and long-term follow-up. Ann Neurol. 1984;15(3):240-244.
24. Terrence CF, Fromm GH. Complications of baclofen withdrawal. Arch Neurol. 1981;38(9):588-589.
25. Zakrzewska JM. Medical management of trigeminal neuropathic pains. Expert Opin Pharmacother. 2010;11(8):1239-1254.
26. Ney JP, Joseph KR. Neurologic uses of botulinum neurotoxin type A. Neuropsychiatr Dis Treat. 2007;3(6):785-798.
27. Aurora SK, Dodick DW, Turkel CC, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia. 2010;30(7):793-803.
28. Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010;30(7):804-814.
29. Wu C, Xie N, Lian Y, et al. Central antinociceptive activity of peripherally applied botulinum toxin type A in lab rat model of trigeminal neuralgia. Springerplus. 2016;5:431.
30. Wu CJ, Lian YJ, Zheng YK, et al. Botulinum toxin type A for the treatment of trigeminal neuralgia: results from a randomized, double-blind, placebo-controlled trial. Cephalalgia. 2012;32(6):443-450.
31. Zhang H, Lian Y, Ma Y, et al. Two doses of botulinum toxin type A for the treatment of trigeminal neuralgia: observation of therapeutic effect from a randomized, double-blind, placebo controlled trial. J Headache Pain. 2014;15:65.
32. Shehata HS, El-Tamawy MS, Shalaby NM, Ramzy G. Botulinum toxin type A: could it be an effective treatment option in intractable trigeminal neuralgia? J Headache Pain. 2013;14:92.
33. Mendlik MT, Uritsky TJ. Treatment of neuropathic pain. Curr Treat Options Neurol. 2015;17(12):50.
34. Lechin F, van der Dijs B, Lechin ME, et al. Pimozide therapy for trigeminal neuralgia. Arch Neurol. 1989;46(9):960-963.
35. Chaudhry P, Friedman DI. Intravenous lidocaine treatment in classical trigeminal neuralgia with concomitant persistent facial pain. Headache. 2014;54(8):1376-1379.
36. Cheshire WP. Fosphenytoin: an intravenous option for the management of acute trigeminal neuralgia crisis. J Pain Symptom Manage. 2001;21(6):506-510.
37. Bajwa Z, Ho CC, Khan SA. Trigeminal neuralgia. UpToDate. www.uptodate.com/contents/trigeminal-neuralgia. Accessed August 24, 2016.
To comment on this article, contact rdavidson@uspharmacist.com.





