US Pharm. 2012;37(9):56-60.
Uncomplicated urinary tract infections (UTIs) are one of the most common reasons for antibiotic use among otherwise healthy women.1 In 2011, the Infectious Diseases Society of America (IDSA) published an update to its clinical practice guidelines for the treatment of uncomplicated UTIs (i.e., cystitis).1 Twelve years had passed since the original guidelines were published, and not surprisingly, there have been a number of changes in treatment recommendations. Such a shift in current practice warrants review of the appropriate diagnosis and treatment of this common women’s health issue.
Pathophysiology
UTIs develop by either ascending or descending bacterial invasion into the urinary tract. The more common mode of infection is the ascending pathway, where fecal flora gain access to the urinary tract via colonization of the urethra. Rarely, a UTI occurs by way of the descending pathway. Descending infections are the result of hematogenous spread of bacteria from a primary source located elsewhere in the body.2 By far, the most common uropathogen identified in uncomplicated UTIs is Escherichia coli, accounting for about 85% of all cases. The remaining 15% are caused primarily by Staphylococcus saprophyticus and Klebsiella and Proteus species.1
Signs and symptoms of a UTI vary depending on the extent of the infection. Lower UTIs can involve the urethra, bladder, and/or prostate (in men), and tend to present with localized symptoms such as dysuria, urinary frequency, urgency, and suprapubic pain or heaviness.3 A UTI extending to the ureter or kidneys (i.e., pyelonephritis) often involves more systemic signs and symptoms, such as leukocytosis, fever, chills, abdominal pain, flank pain, and nausea/vomiting. Certain patient populations may present atypically. For example, elderly patients are less likely to have urinary symptoms and more likely to present with altered mental status, changes in eating habits, and gastrointestinal complaints.2
Diagnosis
There are a number of diagnostic tools that can be utilized to confirm a diagnosis of UTI. In the outpatient setting, the most convenient tool is the urine dipstick, which provides two important markers for UTI detection—leukocyte esterase and nitrite. Leukocyte esterase is indicative of white blood cells in the urine (pyuria). Nitrite indicates bacteriuria and has a higher specificity for UTI than does leukocyte esterase (95%-98% versus 59%-96%).4 However, the sensitivity is limited by bacteria that do not reduce nitrate, such as S saprophyticus and Enterococcus and Pseudomonas species. The most reliable method for confirming UTI is the urine culture. The preferred method for collection is the midstream clean catch, as it is the least invasive. The traditional cut-off for significant bacteriuria in this case is 105 CFU/mL, though some sources cite 102 CFU/mL as diagnostic for a symptomatic patient.2,3
UTIs are classified as either complicated or uncompli-cated. What distinguishes a complicated UTI is the presence of a structural or functional abnormality in the urinary tract. By default, infections in men, children, and pregnant women are considered complicated, as most of these cases involve some type of urologic abnormality. Other characteristics that denote a complicated infection include immunosuppressive conditions, diabetes, catheterization, renal transplantation, and neurogenic bladder.1-3 Consequently, uncomplicated UTIs occur in otherwise healthy, adult, nonpregnant females.
Lower UTI Treatment
In the original IDSA guidelines from 1999, the recommended treatment for uncomplicated lower UTI in the United States was primarily trimethoprim-sulfamethoxazole (TMP-SMX).5 Since that time, new clinical data and a greater emphasis on the potential for antibiotics to propagate antimicrobial resistance (i.e., “collateral damage”) has led to a number of changes in the treatment recommendations within the guideline update (TABLE 1).1 In addition to TMP-SMX, nitrofurantoin and fosfomycin are now also considered appropriate empiric treatment options due to their reasonable efficacy and low risk for collateral damage.1 Fluoroquinolones and beta-lactams remain alternative treatment options due to concerns for antimicrobial resistance and inferior efficacy, respectively.
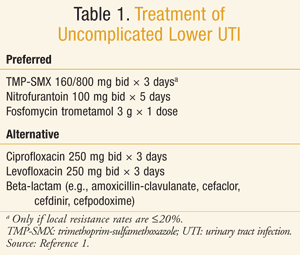
TMP-SMX: Overall, clinical trials for UTIs evaluate two primary outcomes, the resolution of bacteriuria (microbiological cure) and the resolution of symptoms (clinical cure). Several studies support the efficacy of TMP-SMX in uncomplicated cystitis, with an estimated clinical cure rate of 93%.1 Moreover, TMP-SMX maintains reasonable efficacy at resistance rates of 14% to 15%. In one trial, TMP-SMX was noninferior to ciprofloxacin despite a resistance rate of 15%, with a clinical cure rate of 86%.6 Another trial comparing TMP-SMX to nitrofurantoin had similar resistance and a clinical cure rate for TMP-SMX of 79%.7 Not surprisingly, patients treated with TMP-SMX with a uropathogen sensitive to the antibiotic had a much higher clinical cure rate than those with a resistant organism. However, it is worth noting that the clinical cure rate was still 41% in those patients with a TMP-SMX resistant pathogen, likely reflecting spontaneous resolution via host defense mechanisms.
One advantage that TMP-SMX has over other antibiotics is sufficient evidence from clinical, in vitro, and mathematical modeling data to support a threshold of 20% resistance below which the antibiotic is still considered appropriate for empiric use.1 This recommendation may be more practical to implement in the hospital setting, as community antibiograms (antibiotic sensitivity reports) are often not available, and the IDSA guidelines caution against extrapolating hospital antibiograms to resistance patterns in the community. However, one study examining E coli antimicrobial resistance among outpatient urinary isolates revealed several U.S. regions where resistance to TMP-SMX exceeded 20%,8 which calls into question the appropriateness of this antibiotic for empiric treatment in the outpatient setting.
There are several pitfalls to using TMP-SMX. Intolerance to sulfa medications is fairly common, with about 3% of hospital inpatients experiencing a drug rash.9 Less common but still of concern is drug hypersensitivity syndrome, which can involve hematologic abnormalities, renal dysfunction, and life-threatening skin reactions such as Stevens-Johnson syndrome. Renal insufficiency is another issue that may prohibit the use of this agent, primarily due to concern for causing hyperkalemia. For patients on warfarin, TMP-SMX poses a significant interaction via both alteration in protein binding and inhibition of warfarin metabolism, and avoidance of even short-term use may be advisable to avoid potentially dramatic prolongation of the international normalized ratio (INR).
Nitrofurantoin: In the previous IDSA guidelines, nitrofurantoin had scant evidence to support its use in uncomplicated cystitis. Over the past decade, however, a number of clinical trials have revealed excellent microbiological and clinical cure rates with this agent, with an overall estimated clinical cure rate of 93%.1 Traditionally, nitrofurantoin has been used for a total of 7 days, but recent literature indicates a 5-day course to be noninferior to TMP-SMX, with a clinical cure rate of 84%.7 Though clinical benefit data are lacking at varying levels of resistance, U.S. surveillance data indicate E coli resistance to nitrofurantoin at 0% to 5%.8
Pitfalls associated with nitrofurantoin use are mostly related to loss of efficacy in the setting of renal dysfunction. As creatinine clearance (CrCl) declines, urinary excretion of nitrofurantoin is reduced, with little to no excretion occurring when the CrCl falls below 20 mL/min.10 However, there is minimal evidence to delineate the level of renal dysfunction that negates the drug’s clinical efficacy. Several references recommend avoiding nitrofurantoin in patients with a CrCl less than 50 to 60 mL/min due to an increased risk of neurotoxicity and pulmonary toxicity.11,12 Whether or not this is a relevant concern for the short courses used in UTIs is highly debatable, as these rare toxicities are typically seen after accumulation from chronic use.13
Fosfomycin: Fosfomycin is a phosphonic acid derivative and currently the only antibiotic in its class. A small amount of evidence suggests it has comparable clinical efficacy to nitrofurantoin (90% vs. 95%), although with a lower microbiological cure rate (78% vs. 86%).14 Its convenience as a single-dose regimen makes it a particularly attractive treatment option from a medication adherence perspective.
The main drawbacks with fosfomycin are related to cost and availability. Despite its being the shortest treatment for UTI, it is the most expensive at around $40 to $50 for a single dose. Moreover, with UTI as its only indication in the U.S. and the very recent endorsement of its use by the IDSA, the availability of fosfomycin at community pharmacies is variable. Lastly, susceptibility testing of this antibiotic is not routinely performed, which makes surveillance of resistance rates a challenge. However, E coli resistance to fosfomycin in Europe has remained low despite frequent use of this agent,15 suggesting that routine testing may not be required.
Fluoroquinolones: Numerous trials have demonstrated the efficacy of fluoroquinolones at producing both microbiological and clinical cure in the treatment of uncomplicated lower UTI.1 With ciprofloxacin and levofloxacin both available as generic formulations, this antibiotic class represents an efficacious and inexpensive treatment option. Despite these advantages, the IDSA has recommended reserving use of these agents as an alternative rather than a preferred treatment option due to their high propensity for collateral damage. Fluoroquinolone use is directly correlated with fluoroquinolone resistance, and hospital resistance rates are on the rise. One study examining hospital resistance rates across 10 years found an overall 25% relative decline in Pseudomonas aeruginosa susceptibility and a 7% decline in E coli susceptibility to fluoroquinolones.16 In the community setting, U.S. surveillance data indicate that overall E coli resistance to fluoroquinolones is fairly low (~5%).8 However, caution is warranted in interpreting national resistance rates due to significant regional variation. In fact, the same surveillance study found fluoroquinolone resistance rates at 11% and 20% in the mid-Atlantic and west south-central regions, respectively.
There is also a concern for developing resistance to nonquinolone antibiotic classes. For example, fluoroquinolone exposure is an independent risk factor for extended-spectrum beta-lactamase (ESBL)–producing E coli,17 and it has also been associated with methicillin-resistant Staphylococcus aureus (MRSA) isolation.18 As a result, it is recommended to reserve the use of this class for more serious infections for which broad-spectrum coverage is warranted.
Beta-lactams: There is a lack of high-quality evidence with beta-lactams in the treatment of uncomplicated lower UTI. Most trials are either underpowered or use an inappropriate comparator arm. Studies to date of reasonable quality suggest inferiority of beta-lactams to fluoroquinolones.1 Though a small trial comparing cefpodoxime to TMP-SMX suggested comparable cure rates,19 a more recent trial with this cephalosporin resulted in inferior clinical cure rates compared to ciprofloxacin (82% vs. 93%).20 Additionally, there are similar concerns for collateral damage with the third-generation cephalosporins as for fluoroquinolones, particularly with regard to ESBL resistance.17 First-generation cephalosporins, on the other hand, may have a lower propensity for collateral damage, and clinical trials to explore their efficacy in uncomplicated UTI are needed.
Pyelonephritis Treatment
The IDSA guidelines segregate antibiotic recommendations for pyelonephritis into outpatient and inpatient treatment (TABLES 2 and 3). Regardless of the location of treatment, a urine culture should always be sent to evaluate the appropriateness of empiric therapy and allow for streamlining when possible. For outpatient treatment, the fluoroquinolones have the most data to support empiric use.1 This antibiotic class is the only one endorsed by the guidelines for empiric outpatient treatment. There is a paucity of literature with regard to alternative agents.
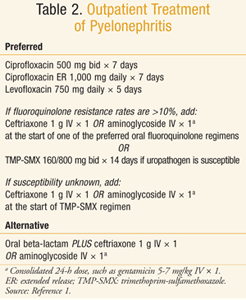
One study found TMP-SMX to be inferior to ciprofloxacin (clinical cure of 83% vs. 95%).21 However, the cure rate for TMP-SMX specifically in patients with susceptible isolates was close to that of ciprofloxacin (92%), indicating TMP-SMX as an acceptable treatment option when the pathogen is known to be sensitive. TMP-SMX–resistant isolates were much less likely to be effectively treated with TMP-SMX, with a clinical cure rate of only 35%. However, patients in the TMP-SMX arm who received one dose of ceftriaxone at the start of treatment had significantly higher microbiological cure rates, and so it is recommended to give a one-time dose of an IV antibiotic when TMP-SMX is used empirically.21 Similarly, it is recommended to administer one IV antibiotic dose in addition to treatment with an oral fluoroquinolone when local resistance rates exceed 10%, though this recommendation is based solely on expert opinion.
Since the previous guidelines, no new clinical literature has been published examining the role of beta-lactams in pyelonephritis. Thus, current recommendations are based on limited and outdated literature, mostly with aminopenicillins (e.g., amoxicillin). Because these data have demonstrated inferior efficacy and higher relapse rates than standard therapy, beta-lactams remain an alternative treatment option.1
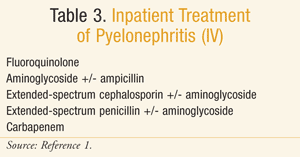
Inpatient treatment is reserved for those patients with severe pyelonephritis and/or the inability to tolerate oral medications. Due to the absence of clinical literature, recommendations for inpatient treatment are largely based on expert opinion, and include a wide variety of broad-spectrum IV antimicrobials (TABLE 3). In this case, local resistance rates and individual patient risk factors for drug-resistant pathogens should be taken into account when determining an appropriate empiric agent.1
Conclusion
While the advent of new IDSA guidelines has broadened the treatment options available for lower UTI in women, each antibiotic has its own advantages and pitfalls, requiring therapy tailored to the individual patient. When possible, fluoroquinolones should be avoided to minimize the potential for collateral damage. Evidence-based treatment options for pyelonephritis remain limited, underscoring the importance of utilizing a hospital or community antibiogram, when available, to guide empiric treatment.
REFERENCES
1. Gupta K, Hooton TM, Naber KG, et al. International
clinical practice guidelines for the treatment of acute uncomplicated
cystitis and pyelonephritis in women: a 2010 update by the Infectious
Diseases Society of America and the European Society for Microbiology
and Infectious Diseases. Clin Infect Dis. 2011;52:e103-e120.
2. Dipiro JT, Talbert RL, Yee GC, et al. Pharmacotherapy: A Pathophysiologic Approach. 7th ed. New York, NY: The McGraw-Hill Companies; 2008:1899-1910.
|3. Lane DR, Takhar SS. Diagnosis and management of urinary tract infection and pyelonephritis. Emerg Med Clin N Am. 2011;29:539-552.
4. Pappas PG. Laboratory in the diagnosis and management of urinary tract infections. Med Clin North Am. 1991;75:313-325.
5. Warren JW, Abrutyn E, Hebel JR, et al. Guidelines for
antimicrobial treatment of uncomplicated acute bacterial cystitis and
acute pyelonephritis in women. Clin Infect Dis. 1999;29:745-758.
6. Arredondo-Garcia JL, Figueroa-Damian R, Rosas A, et al.
Comparison of short-term treatment regimen of ciprofloxacin versus
long-term treatment regimens of trimethoprim/sulfamethoxazole or
norfloxacin for uncomplicated lower urinary tract infections: a
randomized, multicentre, open-label, prospective study. J Antimicrob Chemother. 2004;54:840-843.
7. Gupta K, Hooton TM, Roberts PL, Stamm WE. Short-course
nitrofurantoin for the treatment of acute uncomplicated cystitis in
women. Arch Intern Med. 2007;167:2207-2212.
8. Zhanel GG, Hisanaga TL, Laing NM, et al. Antibiotic resistance in Escherichia coli outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents. 2006;27:468-475.
9. Ho JM, Juurlink DN. Considerations when prescribing trimethoprim-sulfamethoxazole. Can Med Assoc J. 2011;183:1851-1858.
10. Goff JB, Schlegel JU, O’Dell RM. Urinary excretion of
nalidixic acid, sulfamethizole and nitrofurantoin in patients with
reduced renal function. J Urol. 1968;99:371-375.
11. Hanlon JT, Aspinall S, Semla T, et al. Consensus
guidelines for oral dosing of primarily renally cleared medications in
older adults. J Am Geriatr Soc. 2009;57:335-340.
12. Bennett WM, Aronoff GR, Golper TA, et al. Drug Prescribing in Renal Failure. Philadelphia, PA: American College of Physicians; 1987.
13. Goemaere NN, Grijm K, van Hal PT, den Bakker MA. Nitrofurantoin-induced pulmonary fibrosis: a case report. J Med Case Reports. 2008;2:169-173.
14. Stein GE. Comparison of single-dose fosfomycin and a
7-day course of nitrofurantoin in female patients with uncomplicated
urinary tract infection. Clin Ther. 1999;21:1864-1872.
15. Naber KG, Schito G, Botto H, Palou J, et al.
Surveillance study in Europe and Brazil on clinical aspects and
antimicrobial resistance epidemiology in females with cystitis (ARESC):
implications for empiric therapy. Eur Urol. 2008;54:1164-1175.
16. Zervos MJ, Hershberger E, Nicolau DP, et al.
Relationship between fluoroquinolone use and changes in susceptibilities
to fluoroquinolones of selected pathogens in 10 United States teaching
hospitals, 1991-2000. Clin Infect Dis. 2003;37:1643-1648.
17. Rodriguez-Bano J, Alcala JC, Cisneros JM, et al. Community infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Arch Intern Med. 2008;168:1897-1902.
18. Weber SG, Gold HS, Hooper DC, et al. Fluoroquinolones and the risk for methicillin-resistant Staphylococcus aureus in hospitalized patients. Emerg Infect Dis. 2003;9:1415-1422.
19. Kavatha D, Giamarellou H, Alexiou Z, et al.
Cefpodoxime-proxetil versus trimethoprim-sulfamethoxazole for short-term
therapy of uncomplicated acute cystitis in women. Antimicrob Agents Chemother. 2003;47:897-900.
20. Hooton TM, Roberts PL, Stapleton AE. Cefpodoxime vs
ciprofloxacin for short-course treatment of acute uncomplicated
cystitis. JAMA. 2012;307:583-589.
21. Talan DA, Stamm WE, Hooton TM, et al. Comparison of
ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for
acute uncomplicated pyelonephritis in women: a randomized trial. JAMA. 2000;283:1583-1590.
To comment on this article, contact rdavidson@uspharmacist.com.





