US Pharm. 2023;48(12):8-12.
ABSTRACT: The liver is a vital organ that performs crucial metabolic and detoxifying functions. Drug-induced liver injury (DILI) is a complex condition resulting from the adverse effects of prescription and OTC medications. DILI, which has intricate mechanisms involving mitochondrial dysfunction and immune response, can be classified into direct and idiosyncratic subtypes. The diagnosis of DILI can be challenging, often requiring exclusion of other liver diseases. DILI outcomes range from reversible liver injury to life-threatening acute liver failure and chronic liver injury, highighting the importance of early recognition and intervention. Armed with specialized knowledge, pharmacists play a key role in educating patients, promoting lifestyle modifications, and ensuring safe medication use to prevent DILI and prioritize liver health in the community.
The liver, one of the human body’s largest internal organs, plays a pivotal role in metabolism and detoxification. Among its essential functions, the liver produces bile, cholesterol, and specialized proteins that aid in fat transport. Additionally, this vital organ is a critically important player in the process of eliminating drugs and toxins. Notably, the liver possesses remarkable regenerative abilities, allowing it to recover from damage in certain circumstances. This resilience is not without limits, however, as the liver can incur irreparable harm that is often attributed to medication-induced liver injury, also known as drug-induced liver injury (DILI). DILI can result from a wide range of medications encompassing prescription, OTC, and herbal products.1 Typically, DILI manifests within 3 to 6 months following exposure to the causative agent.2 Although DILI is not a common condition—it occurs in 14 to 19 cases per 100,000 individuals annually—its relevance to today’s population, particularly in recent years, emphasizes the importance of understanding its nature and potential consequences.1
Classification
A closer examination of DILI reveals distinct subtypes, each characterized by unique features, and further categorization based on the type of injury unveils its intricate diversity. DILI can be categorized into two primary subtypes: direct (intrinsic) and idiosyncratic. Direct DILI occurs when the injury is dose-dependent, inherent, and susceptible, highlighting the importance of adhering to recommended medication doses. Idiosyncratic DILI refers to a type of liver damage caused by certain medications that occurs unpredictably and is not directly related to the drug’s dose or duration of use; it is characterized by varying clinical presentations and is often challenging to anticipate or prevent, making it a complex form of DILI. Idiosyncratic DILI is a rare occurrence, with an annual rate of approximately 60,000 cases in the United States, and it is often associated with atypical immune responses.1
Idiosyncratic DILI can be further classified based on the specific type of injury involved, resulting in three main categories: hepatocellular, cholestatic, and mixed injury. Hepatocellular injury is characterized by elevated levels of alanine aminotransferase (ALT) and aspartate aminotransferase, which are indicative of a severe prognosis that may necessitate a liver transplant. Cholestatic injury, which involves the obstruction of bile ducts and choledocholithiasis, has subtypes including bland cholestasis, cholestatic hepatitis, and acute cholestasis and is marked by elevated alkaline phosphatase (ALP) and gamma-glutamyltransferase levels. Mixed injury combines elements of hepatocyte necrosis, inflammation, and elevated ALT and ALP levels, closely resembling the broader spectrum of DILI.3
Liver-Injury Information Resources
Pharmacists must maintain continuous access to comprehensive drug information because of the potential for any prescription to trigger DILI, resulting in severe hepatic damage. Having expertise in drug information enables pharmacists to streamline the analysis and interpretation of extensive data, ultimately leading to optimal patient care. A particularly invaluable data set for this purpose is the FDA Adverse Events Reporting System (FAERS). This resource provides key data on liver injury, including case counts categorized by year of receipt (FIGURE 1). By mid-2023, 800 cases had been reported, indicating a consistent annual increase in case numbers since 2022. From 2012 to the present, a total of 20,115 incidents have been documented, with 19,793 classified as critical.4 The FAERS also identifies the drugs more frequently implicated in DILI (FIGURE 2).
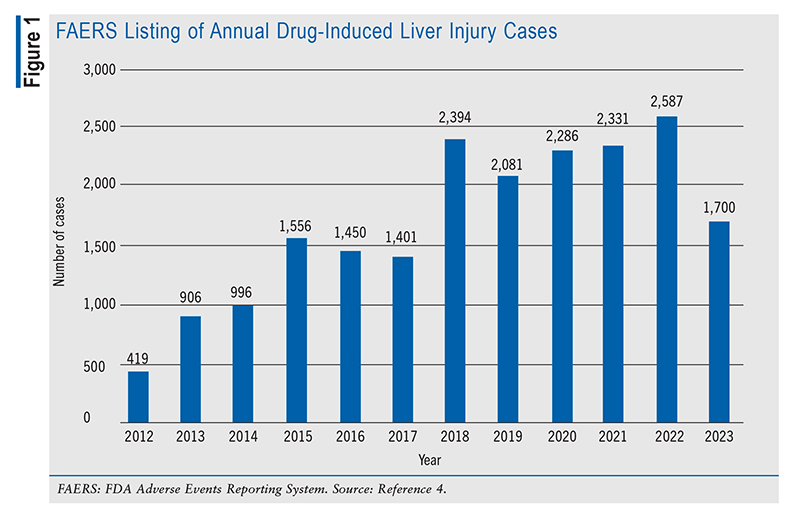
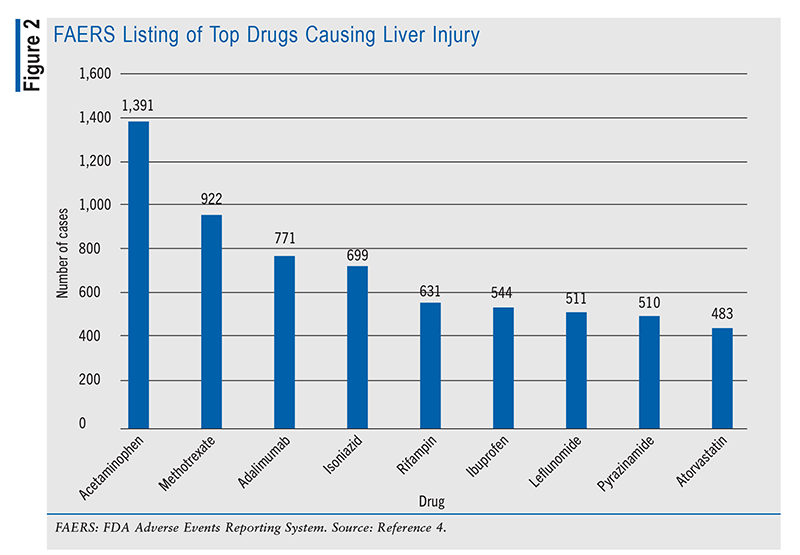
Additionally, the National Institutes of Health’s LiverTox database offers extensive information on drugs associated with liver-related injuries. This valuable resource enables pharmacists to significantly enhance their efforts to prevent liver complications by diligently monitoring medications for adverse effects and educating patients on their correct use.4 Equipped with insights gleaned from the FAERS and LiverTox resources, pharmacists can play a pivotal role in patient care.
Mechanisms
The occurrence of DILI can be attributed to various pathways of injury. During the phases of drug metabolism (phases I-II), there are instances wherein bioactive products can disrupt mitochondrial function, potentially leading to hepatocyte necrosis. These disruptions may involve mechanisms such as heightened activation of reactive oxygen species, depletion of adenosine triphosphate, and inhibition of the mitochondrial respiratory chain.5
Another mechanism of injury is an immune-mediated response, which can be identified by the time from drug administration to the onset of visible liver injury. Bioactive products from medications can interact with the major histocompatibility complex, provoking an immune response against hepatocytes. Immune-mediated DILI can manifest after multiple administrations of the offending medication and may result in a severe reaction upon reexposure.5
Risk Factors
Although there are no overarching all-cause risk factors that universally trigger DILI, several characteristics increase the likelihood of its occurrence. One significant contributing factor is age. Because drug clearance becomes increasingly impaired with advancing years, older patients are more susceptible to developing DILI. Women have been noted to be at higher risk for autoimmune hepatitis–associated DILI, although the impact is mixed. A history of alcohol consumption significantly increases the risk of DILI. Finally, the presence of comorbidities can also induce DILI; chronic inflammation can activate the adaptive immune system, further exacerbating the progression of DILI. Although chronic liver diseases have been assumed to be risk factors, the evidence supporting this link is not particularly robust.6
Diagnosis
Diagnosing DILI is challenging, as it can be mistaken for various other diseases, such as cytomegalovirus, viral hepatitis, biliary diseases, nonfatty alcoholic liver disease, alcohol abuse, autoimmune liver disease, and hereditary conditions.2 Clinical scales are available to assist in the diagnosis of DILI. The Structured Expert Opinion Process is considered the most effective tool, surpassing older methods, such as the Roussel Uclaf Causality Assessment Method (RUCAM); however, RUCAM is still commonly used because of its accessibility.7
It is particularly difficult to diagnose DILI because there are no specific biomarkers to confirm the condition. Clinical symptoms may include loss of appetite, increased fatigue, abdominal pain, rash, ascites, jaundice, nausea, pruritus, and fever. However, some patients may be asymptomatic. Hy’s law may be used to identify cases of hepatocellular injury involving elevated ALT and jaundice, which carry a 10% to 50% mortality rate. This method, which is employed by the FDA, assesses hepatotoxicity rates for new medications. Another approach involves laboratory findings that indicate a threefold elevation in ALT relative to the upper limit of normal (ULN), although this is not the most accurate diagnostic method.8
Causative Agents
A wide range of medications can impact the liver through either direct or idiosyncratic mechanisms, posing complex challenges for clinicians and patients. Whereas some drugs exhibit predictable and dose-dependent hepatotoxicity, others are unpredictable by nature.9 For instance, acetaminophen is capable of causing direct hepatotoxicity. Its metabolism can generate an electrophilic metabolite known as N-acetyl-p-benzoquinone imine, which reacts with glutathione and binds to cellular proteins, leading to oxidative stress, calcium imbalance, and mitochondrial dysfunction. Liver injury from acetaminophen can be mitigated with the antidote N-acetylcysteine.10
Methotrexate, an immunosuppressive medication used in the treatment of cancer, psoriasis, and rheumatoid arthritis, can result in idiosyncratic DILI. Although the exact mechanism remains unclear, it is assumed to involve the inhibition of DNA and RNA synthesis, resulting in cellular arrest. Failure to discontinue methotrexate can lead to fatal consequences such as fibrosis and cirrhosis. Supplementation with folic acid is recommended to reduce the risk of liver-test abnormalities.11
Isoniazid, which is commonly used to treat tuberculosis, exhibits dose-related toxicity, with injury mechanisms linked to increased toxic intermediate accumulation. The symptoms of isoniazid-induced DILI, including jaundice, weakness, nausea, and loss of appetite, signal when discontinuation of the medication may be necessary.12 Another tuberculosis medication, rifampin, is associated with idiosyncratic DILI. Injury is presumed to result from toxic metabolites that can directly affect the liver or trigger an immune response. Rifampin can lead to outcomes such as acute liver failure (ALF) and death, emphasizing the importance of monitoring for liver disease in patients who are undergoing tuberculosis treatment.13
Ibuprofen, a widely used nonsteroidal anti-inflammatory drug, can also have serious effects, although the exact mechanism of injury remains unclear. Ibuprofen is often associated with a toxic metabolite byproduct that can provoke an immune response. Prompt recovery can occur upon discontinuation of the drug, but without treatment, conditions such as vanishing bile duct syndrome and fulminant liver failure may develop. Chronic exposure to ibuprofen after a patient experiences hepatic impairment can also result in DILI.14
As a class, statins result in various degrees of liver injury, with atorvastatin being notably hepatotoxic.15 The precise mechanism of statin-induced injury remains uncertain, but it has been suggested that mitochondrial damage may be involved.16 This potential idiosyncratic injury is believed to occur with the highest doses used. For atorvastatin-induced injury, management involves monitoring ALT levels (ensuring that they do not exceed 10 times the ULN) and discontinuing the medication, and recovery is expected within 1 to 4 months.17
Outcomes
DILI encompasses a range of outcomes, from potential reversibility to severe and sometimes life-threatening consequences, making it imperative that healthcare providers and patients understand these possible scenarios and the need for timely intervention. Regarding reversibility, the identification and prompt discontinuation of the causative medication upon recognition of liver injury significantly increase the likelihood of complete liver-function recovery. However, rapid discontinuation is paramount, as prolonged use of the causative agent escalates the risk of irreversible liver damage. DILI can progress to ALF, a severe and life-threatening condition characterized by rapid deterioration of liver function.14 Although rare, ALF poses a substantial risk of morbidity and mortality. In the U.S., DILI—particularly its idiosyncratic form—is the primary cause of ALF. Given the unpredictable nature of idiosyncratic DILI, early recognition and intervention are critical for curbing its progression to ALF.
DILI can also result in chronic liver injury, a condition that manifests when DILI persists over an extended period because of the patient’s ongoing exposure to hepatotoxic medications.18 Specific drugs, such as amoxicillin/clavulanate, diclofenac, and halothane, are linked to this chronic form of DILI. Prolonged exposure to these substances can exacerbate liver injury, potentially resulting in complications and necessitating continuous monitoring and management.
Another potential consequence of severe and prolonged DILI is drug-induced cirrhosis. This condition involves extensive liver fibrosis and scarring, leading to impaired liver function and increasing the risk of complications.19 Although drug-induced cirrhosis is relatively rare compared with other DILI outcomes, the potential for its development underscores the importance of early recognition, discontinuation of the causative agent, and comprehensive long-term management.
The Pharmacist’s Role
Utilizing their vast knowledge of drug information, pharmacology, and medication management, pharmacists play a pivotal role in safeguarding patients’ safety and well-being concerning medication-induced liver injury. Pharmacists possess the skills to minimize the risks associated with DILI by identifying and addressing factors that may adversely affect liver health.
Pharmacists can offer valuable counseling in various pertinent areas, such as limiting or avoiding alcohol consumption, since excessive alcohol use frequently results in conditions such as alcoholic hepatitis and hepatic steatosis. Although both of these conditions may be reversible if detected early, prolonged damage may impair liver-cell function. Pharmacists can also offer guidance on weight loss, which can help patients reduce liver fat, inflammation, and scarring. Pharmacists should emphasize safety practices to prevent conditions such as viral hepatitis, including avoiding exposure to contaminated foods and water and practicing safe sex. Chronic infections can ultimately lead to cirrhosis.
Patients should receive regular liver-function testing to monitor for elevations or abnormalities, and Asian and Pacific Islander individuals born outside the U.S. should undergo screening every 6 months for hepatitis B.20 By counseling on lifestyle modifications, encouraging safety practices, and promoting regular liver-function testing, pharmacists emerge as frontrunners in preventing DILI and ensuring the overall well-being of their patients. Their proactive approach to patient education and advocacy emphasizes their dedication to liver health in the community.
Conclusion
DILI poses a multifaceted challenge within the realm of pharmacotherapy and patient care. Ultimately, the ability to navigate the complexities of DILI highlights the commitment of healthcare professionals to prioritizing patient safety, enhancing medication-management protocols, and ensuring that the benefits of pharmacotherapy are realized while minimizing potential risks to liver health. In this ongoing effort, pharmacists and other healthcare professionals can collaboratively support and promote the well-being of their patients.
REFERENCES
1. Fontana RJ, Liou I, Reuben A, et al. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology. 2023;77(3):1036-1065.
2. Francis P, Navarro VJ. Drug-induced hepatotoxicity. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-.
3. Kullak-Ublick GA, Andrade RJ, Merz M, et al. Drug-induced liver injury: recent advances in diagnosis and risk assessment. Gut. 2017;66(6):1154-1164.
4. FDA Adverse Events Reporting System (FAERS) Public Dashboard. Drug-induced liver injury. https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis. Accessed September 26, 2023.
5. Yuan L, Kaplowitz N. Mechanisms of drug-induced liver injury. Clin Liver Dis. 2013;17(4):507-518.
6. Li X, Tang J, Mao Y. Incidence and risk factors of drug-induced liver injury. Liver Int. 2022;42(9):1999-2014.
7. Tillmann HL, Suzuki A, Barnhart HX, et al. Tools for causality assessment in drug-induced liver disease. Curr Opin Gastroenterol. 2019;35(3):183-190.
8. Andrade RJ, Robles-Díaz M. Diagnostic and prognostic assessment of suspected drug-induced liver injury in clinical practice. Liver Int. 2020;40(1):6-17.
9. Teschke R, Danan G. Drug induced liver injury: mechanisms, diagnosis, and clinical management. In: Radu-Ionita F, Pyrsopoulos NT, Jinga M, et al, eds. Liver Diseases: A Multidisciplinary Textbook. Cham, Switzerland: Springer Nature Switzerland AG; 2020:95-105.
10. Acetaminophen. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.
11. Methotrexate. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.
12. Isoniazid. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.
13. Rifampin. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.
14. Ibuprofen. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.
15. Chen M, Suzuki A, Thakkar S, et al. DILIrank: the largest reference drug list ranked by the risk for developing drug-induced liver injury in humans. Drug Discov Today. 2016;21(4):648-653.
16. Averbukh LD, Turshudzhyan A, Wu DC, Wu GY. Statin-induced liver injury patterns: a clinical review. J Clin Transl Hepatol. 2022;10(3):543-552.
17. Atorvastatin. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.
18. Wang Q, Huang A, Wang JB, Zou Z. Chronic drug-induced liver injury: updates and future challenges. Front Pharmacol. 2021;12:627133.
19. Clinical course and diagnosis of drug induced liver disease. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.
20. Stanford Medicine Health Care. Liver disease prevention. https://stanfordhealthcare.org/medical-treatments/l/liver-disease-prevention.html. Accessed September 25, 2023.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






