US Pharm. 2021;46(11):44-50.
ABSTRACT: Although it is possible for patients with type 2 diabetes mellitus to achieve glycemic control with lifestyle changes and oral medications, many patients require insulin therapy to achieve adequate glycemic control. Long-acting basal insulins are the cornerstone of all insulin regimens. More novel basal insulins created to exhibit superior pharmacokinetic profiles are now being developed; recently, two novel long-acting basal insulins with superior pharmacokinetic profiles have emerged on the market. Insulin icodec, a once-weekly, ultralong-acting basal insulin analogue is currently in development for the treatment of diabetes. Additionally, biosimilar products are becoming more common and offer more cost-saving options for patients. Pharmacists must be knowledgeable about how novel basal insulins differ from traditional insulin analogues.
Diabetes mellitus (DM) affects more than 34.2 million people in the United States, and approximately one-third of this population has type 2 DM (T2DM) and requires insulin therapy.1 Although it is possible for patients with T2DM to achieve glycemic control with lifestyle changes and oral medications, many patients require insulin therapy to achieve adequate glycemic control. Long-acting basal insulins are the cornerstone of all insulin regimens. Despite advances in formulations such as insulin glargine and insulin detemir, hypoglycemia and weight gain remain significant obstacles to achieving the desired glycemic control with insulin therapy.2 Recently, two novel long-acting basal insulins with superior pharmacokinetic profiles have emerged on the market. Pharmacists must be knowledgeable about how novel basal insulins differ from traditional insulin analogues. This article is an update on the currently available basal insulins.
Role of Basal Insulin in T2DM Treatment
According to the current American Diabetes Association (ADA) Standards of Medical Care in Diabetes, insulin can be started at any point, even upon diagnosis, depending on the patient’s hemoglobin A1C level.3 After failure of lifestyle modifications, metformin should be initiated and titrated to an optimal dosage of 2,000 mg per day to provide enhanced glycemic control as well as the cardiovascular benefits established by the United Kingdom Prospective Diabetes Study.4-6 If the patient’s A1C is >9% or remains above goal after 2 to 3 months on optimally dosed metformin therapy, then dual therapy with any other diabetes agent, including insulin, may be initiated. If a patient’s A1C is >10% upon diagnosis, basal insulin should be initiated in addition to lifestyle modifications and metformin therapy. Prandial coverage with a single dose of bolus insulin at the patient’s largest meal or a glucagon-like peptide-1 receptor agonist may be added if the patient does not reach the A1C goal on basal therapy. After that, if glycemic control still has not been achieved, the patient should be advanced to a full basal-bolus regimen.3 It is important to note that the addition of insulin therapy should not be delayed, as earlier glycemic control has been shown to significantly reduce long-term cardiovascular complications and mortality risk.7
Titration and Dosing
The ADA’s Standards of Medical Care in Diabetes recommends either starting an initial total daily dose of 10 units or using a weight-based total daily dose of 0.1 to 0.2 units/kg.3 Given that T2DM patients may require relatively higher doses than patients with type 1 DM because they have insulin resistance or are overweight, it is often more appropriate to use weight-based dosing.1 Ideally, insulin should be titrated up 10% to 15% once or twice weekly to reach average fasting blood-glucose concentrations <130 mg/dL.8 Conversely, a patient’s insulin dose should be titrated down by 10% if a patient experiences a hypoglycemic event with no addressable cause. Common addressable causes include missing a meal and inappropriate timing of insulin injections relative to meals or lack thereof.3 A treat-to-target schedule may also be used to titrate a patient’s dose based on average fasting blood-glucose concentrations. As a rule, the basal insulin dose may be increased by 2 units for every 20 mg/dL that the patient’s average fasting blood-glucose level is over the recommended goal fasting blood-glucose level (<130 mg/dL per ADA guidelines). For example, if the patient’s average fasting blood-glucose level is 150 mg/dL, the dose could be increased by 2 units. However, it is not recommended to increase the dose by more than 8 units in a single titration.9
Pharmacologic Profiles
Features of an ideal basal insulin include a flat, peakless activity profile to minimize the risk of hypoglycemic events; a long duration of action (>24 hours) to allow for dosing flexibility; minimal intrapatient and interpatient variability to enable predictablity of dose responses; and a minimal risk of weight gain.3
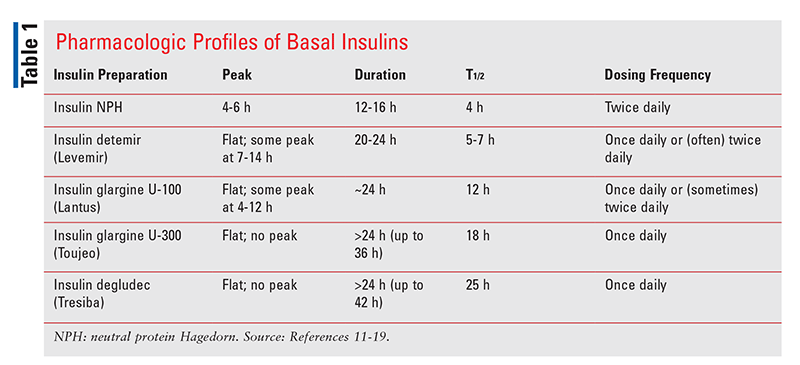
Intermediate-acting insulin neutral protamine Hagedorn (NPH; Novolin N) carries the highest risk of hypoglycemia because it has the most pronounced peak (occurring 4 to 6 hours after administration).10,11 It also has the shortest half-life (12-16 hours) and requires twice-daily dosing.11 Insulin detemir (Levemir) and insulin glargine 100 units/mL (U-100; Lantus) exhibit far fewer pronounced peaks than insulin NPH and have longer half-lives. Their pharmacokinetic profiles are mostly flat, but there is still some peak at 7 to 14 hours for insulin detemir and 4 to 12 hours for Lantus.12,13 Frequency of dosing can vary for both of these agents. Insulin detemir can only be dosed once daily at higher total daily doses of 0.8 units/kg.12,14 However, even at this higher dose, detemir still does not reach a duration of action equal to 24 hours.15 Lantus was considered the only true once-daily basal insulin; however, some patients require twice-daily dosing owing to a waning effect, whereas in other patients twice-daily dosing is necessary because of impaired absorption at doses >1.0 units/kg.16,17 Nonetheless, besides the potential for once-daily dosing, the traditional insulin basal analogues exhibit other advantages over NPH, including lower risks of overall and nocturnal hypoglycemia, less variability, and less weight gain.10
Novel basal insulins were designed to exhibit more features of an ideal basal insulin than traditional insulins. In fact, both insulin glargine 300 units/mL (U-300; Toujeo) and insulin degludec (Tresiba) have flatter profiles than insulin glargine U-100; have relatively lower patient variability compared with traditional basal insulins; and have a longer duration of action >24 hours—up to 36 hours and 42 hours, respectively—that enables true once-daily dosing.18,19
TABLE 1 summarizes the pharmacologic profiles of different basal insulins.
Toujeo and Lantus: Efficacy and Safety
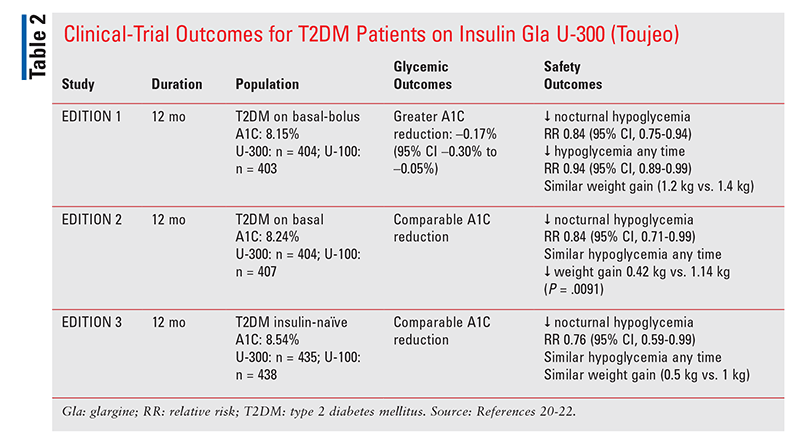
Insulin Glargine U-300 (Toujeo): A series of clinical trials (TABLE 2) compared concentrated insulin glargine U-300 with U-100 (Lantus).20,22 Studies that included only patients with T2DM showed that concentrated U-300 demontrated no difference in A1C reductions compared with U-100 and no difference in rates of overall hypoglycemia, with the exception of patients on basal-bolus regimens.21,22 However, basal-bolus patients on U-300 exhibited a greater A1C reduction of –0.17% (95% CI, –0.30%-0.05%) and less risk of hypoglycemia (relative risk [RR] 0.94; 95% CI, 0.89-0.99) than patients on U-100.20 All study populations had a significantly lower risk of nocturnal hypoglycemia on U-300, ranging from a 16% RR reduction for patients on basal-bolus regimens (RR 0.84; 95% CI, 0.75-0.94) and patients switched to U-300 on basal therapy alone (RR 0.84; 95% CI, 0.71-0.99), and up to a 24% RR in insulin-naïve patients (RR 0.76; 95% CI, 0.59-0.99).21,22 In addition, less weight gain was observed in patients on U-300, with an average of 0.42 kg gained in the U-300 group versus 1.14 kg gained in the U-100 group (P = .0091).21
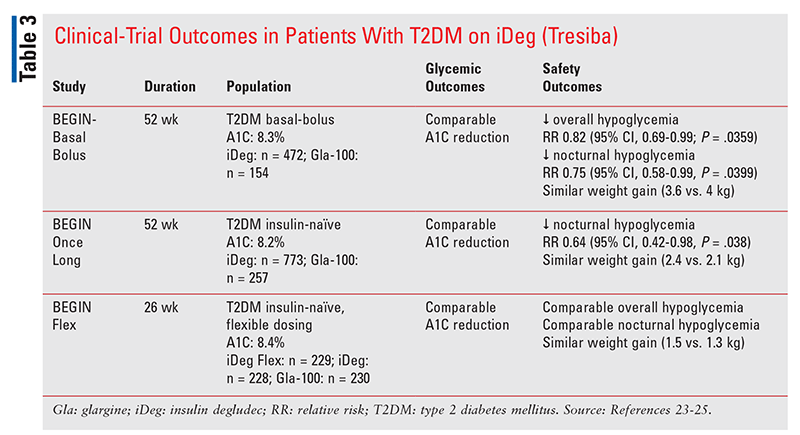
Insulin Degludec (Tresiba): A series of clinical trials compared insulin degludec with Lantus (TABLE 3). Overall, there was no difference in A1C reductions, but patients on insulin degludec had a 25% lower risk of nocturnal hypoglycemia if they were on a basal-bolus regimen (RR 0.75; 95% CI, 0.58-0.99) and a 36% decreased risk if they had not previously been on any insulin (RR 0.64; 95% CI, 0.42-0.98).23,24 Patients on basal-bolus regimens also had a lower overall risk of hypoglycemia with insulin degludec versus Lantus (RR 0.2; 95% CI 0.69-0.99).23 However, findings from the BEGIN FLEX trial are what distinguish insulin degludec from all other basal insulins, including Toujeo. In this study, patients on flexible dosing schedules of insulin degludec were compared with patients on once-daily dosing of either Lantus or Tresiba. Flexible-dosing patients alternated the timing of administration between each dose of Tresiba by 36 to 40 hours one day and then 8 to 12 hours the next throughout the 26-week study period. At the end of the study period, patients in the flexible-dosing group on insulin degludec had comparable A1C reduction, comparable hypoglycemia, and comparable nocturnal hypoglycemia to those on daily doses of insulin glargine U-100 and insulin degludec scheduled every 24 hours.25 Therefore, insulin degludec may be best for patients nonadherent to timing of administration because of erratic work schedules or frequent international or transcontinental travel, as it permits a flexible dosing schedule without compromising safety or efficacy.
Pipeline Agent: Insulin Icodec
Insulin icodec is a once-weekly, ultralong-acting, basal insulin analogue that is currently in development for the treatment of diabetes. This novel approach was developed by reengineering the ultralong oral basal insulin known as OI338.26 The two significant modifications made to OI338 to form insulin icodec consist of the strong, reversible binding to albumin and reduced insulin receptor affinity to slow down clearance and provide the continuous action of the insulin. Recent pharmacokinetic and pharmacodynamic studies show that insulin icodec has a half-life of approximately 196 hours, ideal for a once-weekly injection.27
Two phase II studies of insulin icodec have had encouraging findings. The first study compared insulin icodec with once-daily insulin glargine U-100 in patients who had not previously received insulin treatment. Results revealed that insulin icodec had a glucose-lowering effect and a safety profile similar to insulin glargine U-100.28
In a phase II, multicenter, open-label, treat-to-target clinical trial, U-100 was switched to insulin icodec in patients who were also receiving one or more glucose-lowering medications. The switch from insulin glargine U-100 to insulin icodec was well tolerated and demonstrated improvements in glycemic control. The hypoglycemia risk was not increased for insulin icodec versus insulin glargine U-100.29
The management of diabetes with insulin icodec offers a variety of advantages that ultimately improve glycemic control with less risk of hypoglycemia. Some of these advantages include a reduced number of injections, increased overall patient adherence, improved quality of life, and decreased treatment burden.30
Role of Biosimilars
Biosimilar insulin products help reduce the overall cost of insulin by fostering more competition on the market. As a result, this can increase patients’ access and adherence to insulin therapy.31
A biosimilar is a biological product that is highly similar, in both structure and function, to an FDA-approved reference product and has been shown in studies to have no clinically meaningful differences in terms of purity, potency, and safety. Minor differences in clinically inactive compounds, such as a buffer or stabilizer, are considered acceptable but are evaluated carefully by the FDA to ensure that the biosimilar product meets approval standards.31 Currently, one biosimilar (Basaglar) is available for Lantus.
Interchangeable biosimilars are the same as biosimilar products, but to receive FDA approval of interchangeability, they must meet additional requirements based on data from further evaluation and testing that show that the biosimilar can produce the same clinical result as the reference product in any given patient. Also, for a product that requires more than one administration, a manufacturer must provide data to assess the risk—in terms of safety and reduced efficacy—of alternating or switching between the products.31 In July 2021, the FDA granted approval of the first interchangeable biosimilar insulin product in the U.S. Semglee, produced by Mylan Pharmaceuticals, was approved to be interchangeable with Lantus. In a phase III, open-label, randomized, parallel-group, noninferiority study comparing the efficacy and safety of Semglee versus the reference drug Lantus, Semglee was shown to be noninferior to Lanus in terms of A1C reduction. Additionally, there were no differences between groups in insulin dosage, self-monitored blood glucose, immunogenicity, or adverse events, including overall hypoglycemia and nocturnal hypoglycemia.32
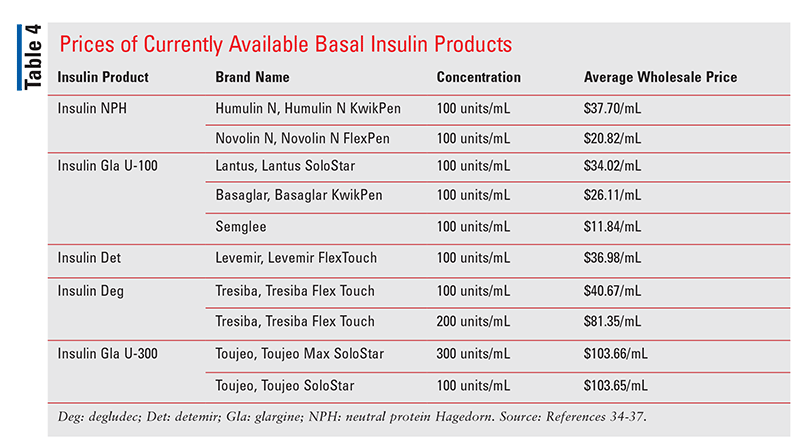
Biosimilars currently marketed in the U.S. typically have an initial price that is 15% to 35% lower than that of the corresponding reference product.33 The Average Wholesale Price of Basaglar and Semglee is currently $26.11/mL and $11.84/mL, respectively, compared with $34.02/mL for the reference product, Lantus. However, even Lantus is more affordable than its current competition compared with other available basal insulin products that do not have biosimilar alternatives. See TABLE 4.34-37
Conclusion
From clinical studies, it is apparent that the novel insulins offer certain advantages over other basal insulins, such as insulin glargine U-100 (Lantus), insulin detemir (Levemir), and insulin NPH (Novolin N, Humulin N). These advantages include longer half-lives and durations of action that allow for true once-daily dosing without a waning effect and an improved safety profile with a decreased risk of nocturnal hypoglycemia and overall hypoglycemia in patients on basal-bolus regimens.18-20,23 However, it is also important to consider affordability before recommending or initiating any of these agents, as cost can significantly increase a patient’s likelihood of nonadherence.38 In addition, patients with T2DM often require larger amounts of insulin because of insulin resistance and obesity. Fortunately, cost will be less of a barrier to adherence with the increase in market competition and the availability of biosimilar products, such as Basaglar, and the most recently approved interchangeable biosimilar, Semglee. Finally, insulin icodec remains a promising treatment option compared with traditional basal insulin products, as it offers the convenience of once-weekly dosing with fewer injections and less risk of hypoglycemia while lessening the treatment burden in patients with T2DM.
REFERENCES
1. CDC. Prevalence of diagnosed diabetes. www.cdc.gov/diabetes/data/statistics-report/diagnosed-diabetes.html. Accessed August 28, 2021.
2. Owens DR, Matfin G, Monnier L. Basal insulin analogues in the management of diabetes mellitus: what progress have we made? Diabetes Metab Res Rev. 2014;30(2):104-119.
3. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes–2021. Diabetes Care. 2021;44(suppl 1):S111-S124.
4. Scarpello JH. Review: optimal dosing strategies for maximising the clinical response to metformin in type 2 diabetes. Br J Diabetes Vasc Dis. 2001;1(1):28-36.
5. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405-412.
6. Grant PJ. The effects of high- and medium-dose metformin therapy on cardiovascular risk factors in patients with type II diabetes. Diabetes Care. 1996;19(1):64-66.
7. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
8. Petznick A. Insulin management of type 2 diabetes mellitus. Am Fam Physician. 2011;84(2):183-190.
9. Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues versus NPH human insulin in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract. 2008;81(2):184-189.
10. Novolin N (isophane insulin human) package insert. Plainsboro, NJ: Novo Nordisk Inc; November 2019.
11. Lantus (insulin glargine) package insert. Bridgewater, NJ: sanofi-aventis U.S. LLC; January 2021.
12. Levemir (insulin detemir) package insert. Plainsboro, NJ: Novo Nordisk Inc; March 2020.
13. Plank J, Bodenlenz M, Sinner F, et al. A double-blind, randomized, dose-response study investigating the pharmacodynamic and pharmacokinetic properties of the long-acting insulin analog detemir. Diabetes Care. 2005;28(5):1107-1112.
14. Klein O, Lynge J, Endahl L, et al. Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time-action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab. 2007;9(3):290-299.
15. Ashwell SG, Gebbie J, Home PD. Twice-daily compared with once-daily insulin glargine in people with type 1 diabetes using meal-time insulin aspart. Diabet Med. 2006;23(8):879-886.
16. Wang Z, Hedrington MS, Gogtidze Joy N, et al. Dose-response effects of insulin glargine in type 2 diabetes. Diabetes Care. 2010;33(7):1555-1560.
17. Becker RH, Dahmen R, Bergmann K, et al. New insulin glargine 300 Units • mL–1 provides a more even activity proile and prolonged glycemic control at steady state compared with insulin glargine 100 Units • mL–1. Diabetes Care. 2015;38(4):637-643.
18. Heise T, Nosek L, Bøttcher SG, et al. Ultra-long-acting insulin degludec has a flatter, stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14(10):944-950.
19. Riddle MC, Bolli GB, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 1). Diabetes Care. 2014;37(10):2755-2762.
20. Yki-Järvinen H, Bergenstal R, Ziemen M, et al. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (EDITION 2). Diabetes Care. 2014;37(12):3235-3243.
21. Bolli GB, Riddle MC, Bergenstal RM, et al. New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (EDITION 3). Diabetes Obes Metab. 2015;17(4):386-394.
22. Garber AJ, King AB, Del Prato S, et al. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379(9825):1498-1507.
23. Zinman B, Philis-Tsimikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long). Diabetes Care. 2012;35(12):2464-2471.
24. Meneghini L, Atkin SL, Gough SC, et al. The efficacy and safety of insulin degludec given in variable once-daily dosing intervals compared with insulin glargine and insulin degludec dosed at the same time daily: a 26-week, randomized, open-label, parallel-group, treat-to-target trial in individuals with type 2 diabetes. Diabetes Care. 2013;36(4):858-864.
25. Kjeldsen TB, Hubálek F, Hjørringgaard C, et al. Molecular engineering of insulin icodec, the first acylated insulin analog for once-weekly administration in humans. J Med Chem. 2021;64(13):8942-8950.
26. Nishimura E, Pridal L, Glendorf T, et al. Molecular and pharmacological characterization of insulin icodec: a new basal insulin analog designed for once-weekly dosing. BMJ Open Diabetes Res Care. 2021;9(1):e002301.
27. Rosenstock J, Bajaj HS, Janez A, et al. Once-weekly insulin for type 2 diabetes without previous insulin treatment. N Engl J Med. 2020;383(22):2107-2116.
28. Bajaj HS, Bergenstal RM, Christoffersen A, et al. Switching to once-weekly insulin icodec versus once-daily insulin glargine U100 in type 2 diabetes inadequately controlled on daily basal insulin: a phase 2 randomized controlled trial. Diabetes Care. 2021;44(7):1586-1594.
29. Lingvay I, Buse JB, Franek E, et al. A randomized, open-label comparison of once-weekly insulin icodec titration strategies versus once-daily insulin glargine U100. Diabetes Care. 2021;44(7):1595-1603.
30. Kuhlmann M, Schmidt A. Production and manufacturing of biosimilar insulins: implications for patients, physicians, and health care systems. Biosimilars. 2014;4:45-58.
31. FDA. Biosimilar and interchangable products. www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products. Accessed August 30, 2021.
32. Blevins TC, Barve A, Sun B, et al. Efficacy and safety of MYL-1501D versus insulin glargine in patients with type 2 diabetes after 24 weeks: results of the phase III INSTRIDE 2 study. Diabetes Obes Metab. 2019;21(1):129-135.
33. FDA. FDA and FTC announce new efforts to further deter anti-competitive business practices, support competitive market for biological products to help Americans. www.fda.gov/news-events/press-announcements/fda-and-ftc-announce-new-efforts-further-deter-anti-competitive-business-practices-support. Accessed August 30, 2021.
34. Insulin NPH. Lexi-Drugs [online database]. Riverwoods, IL: Lexicomp, Inc. http://online.lexi.com. Accessed September 6, 2021.
35. Insulin glargine. Lexi-Drugs [online database]. Riverwoods, IL: Lexicomp, Inc. http://online.lexi.com. Accessed September 6, 2021.
36. Insulin detemir. Lexi-Drugs [online database]. Riverwoods, IL: Lexicomp, Inc. http://online.lexi.com. Accessed September 6, 2021.
37. Insulin degludec. Lexi-Drugs [online database]. Riverwoods, IL: Lexicomp, Inc. http://online.lexi.com. Accessed September 6, 2021.
38. García-Pérez LE, Alvarez M, Dilla T, et al. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175-194.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





