US Pharm. 2016;41(8):26-30.
ABSTRACT: Urine drug screening is a common way to test for compliance with medications having high abuse potential. False-negatives and false-positives from immunoassays can lead to adverse consequences for patients and providers. By identifying medications that contribute to false-negatives and false-positives, pharmacists decrease misinterpretations from urine drug screens. Unexpected results from urine immunoassays should have a confirmatory gas chromatography–mass spectrometry or a high-performance liquid chromatography test performed. Pharmacists can provide guidance in selecting appropriate drug therapies that are less likely to cause false readings, thus decreasing the need for additional testing.
Urine drug screen (UDS) testing can increase workplace safety, detect drug abuse, monitor patients’ compliance with prescription medications, and assess suspected drug ingestions.1 Thus, these tests are commonly used in clinical practice to support decision-making on the use of high-risk medications. The most frequently used type of UDS is the immunoassay due to its low cost, rapidity of results, and simplicity of use. Immunoassays detect substances above a set threshold using antibodies.1,2 While a useful tool, immunoassays have poor specificity that may lead to false-positive results.1-3 Unexpected results should be confirmed with a second test, such as gas chromatography–mass spectrometry (GC-MS) or high-performance liquid chromatography (HPLC), that is more accurate; however, these tests are costly and require extra time to perform.1-3 Therefore, patient care decisions are often made based on presumed positive or negative immunoassay test results.
Interpreting Test Results
Misinterpretation of UDS results may have adverse consequences for patients, including unwarranted loss of a job, potential criminal charges, loss of qualification from sporting events or rehabilitation programs, potentially improper medical treatment, or loss of trust from healthcare professionals.2,3 Patients who are required to receive random or recurrent UDS testing as part of rehabilitation programs; as a stipulation of employment; for health monitoring, such as for pain management or medication compliance; or for other reasons are at particularly high risk of negative consequences from misinterpreted UDS results.1,4 To decrease the likelihood of misinterpretation, pharmacists can help by identifying medications at high risk for causing false-negatives and false-positives and choosing medications less likely to cause these inaccuracies.
False-Negatives: To aid in interpreting UDS results, pharmacists should acquire a thorough list of all the patient’s prescription, OTC, and herbal medications prior to testing, as well as discuss adherence to medications. When a negative screening result is obtained, pharmacists should carefully consider the potential for a false-negative result, especially for patients receiving UDS testing to assess compliance with a medication regimen or for those exhibiting behaviors or risk factors suggestive of drug abuse or drug dependency.1
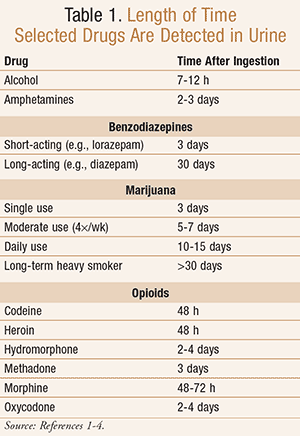
False-negatives can occur when the urine drug concentration is below the threshold level set by the laboratory performing the test.1,2 Dilute urine, the duration of time between ingestion of the drug and time of testing, and the quantity of the drug ingested may affect the occurrence of false-negatives.1-2 While chronic marijuana use will show in the urine for weeks after heavy use, other medications and illicit drugs will only be present for 1 to 4 days, as shown in TABLE 1.1-4
Patients may purposefully attempt to hide positive screening results by adding contaminants to their urine that mask the presence of a drug, such as vinegar, soap, bleach, drain cleaner, eye drops, table salt, or ammonia.5 Additionally, commercial products with the active ingredients peroxide (peroxidase), glutaraldehyde, sodium or potassium nitrite, and pyridinium chlorochromate could be used.5 Changes in urine appearance, color, specific gravity, or pH may indicate the presence of a contaminant and should be checked. Patients may also drink an excessive amount of water (2-4 qt) or use diuretics to purposefully dilute their urine and the urine drug concentration to decrease the chance of detection.5,6
Furthermore, false-negatives may also occur because the UDS is simply unable to detect the agent. For example, UDS tests for benzodiazepines commonly result in false-negatives for agents that have poor cross-reactivity with the assay.7 Most assays for benzodiazepines detect their presence in the urine by testing for nordiazepam and oxazepam, the main metabolites of most benzodiazepines.2 Agents that follow a different metabolic pathway, such as triazolam, alprazolam, clonazepam, and lorazepam, have poor cross-reactivity with the assay due to the absence of these metabolites and thus frequently produce false-negative results.2,7 Therefore, to decrease the need for confirmatory testing, diazepam, oxazepam, and temazepam may be preferred.
Similarly, opiates can be at risk for false-negatives. Most immunoassay tests look for morphine, norcodeine, and codeine; thus morphine, heroin, and codeine can easily be detected. Hydrocodone and hydromorphone are metabolites of codeine and are rarely positive on immunoassay tests. Oxycodone, buprenorphine, and tramadol follow a separate metabolic pathway, and fentanyl may not be detected because it lacks metabolites.1,4 To minimize the need for confirmatory testing, consider using morphine or codeine in high-risk patients.
For patients being treated for attention-deficit/hyperactivity disorder (ADHD), UDS testing may also be recommended. Immunoassays test for amphetamines; thus, amphetamine, dextroamphetamine, and lisdexamfetamine products should return positive results for compliance testing if taken in the last 2 to 3 days. Illicit methamphetamine will also show positive within the amphetamine immunoassay test. However, methylphenidate products do not cross-react with amphetamines and will commonly produce negative results,8 although a false-positive result with methylphenidate has been seen in one pediatric case report.1-2,8 If methylphenidate products are used, a GC-MS test should be routinely administered.
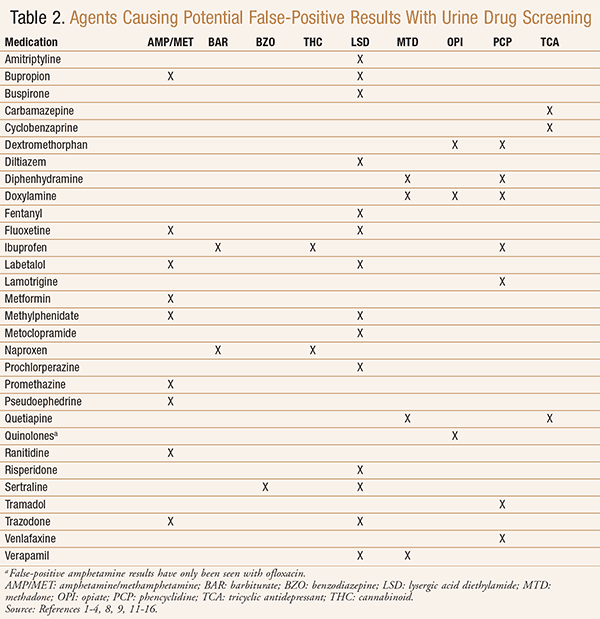
False-Positives: In addition to false-negatives, pharmacists need to consider the potential for false-positive UDS results and be aware of medications that may cause false-positives. TABLE 2 summarizes many medications that have been reported to cause false-positive results with common substances of abuse or tricyclic antidepressants (TCAs).1-4 False-positives can occur when a medication has a cross-reactivity with the immunoassay, often due to a similarity in the structure of the parent medication or one of its metabolites to the tested drug.2 The occurrence of false-positives is mostly affected by the type of immunoassay used and by the particular agent being tested.2
When selecting therapeutic agents for high-risk patients, pharmacists should consider minimizing the use of drugs known to cause false-positive results, if possible. The selection of an appropriate therapeutic agent for a patient depends on numerous factors, such as the effectiveness and adverse-effect profile of the drug; therefore, minimizing the use of medications shown to cause false-positives must be weighed against clinical judgment in product selection. However, for patients undergoing frequent UDS testing, selecting an agent least likely to cause false-positives would be an important consideration to help minimize adverse consequences to patients from potentially misinterpreted results.
Antidepressants
Many of the medications reported to cause false-positive UDS results include a variety of antidepressants, which can be used for various indications. Of the selective serotonin reuptake inhibitors (SSRIs), sertraline has been reported to cause false-positive results for benzodiazepines and lysergic acid diethylamide (LSD),1-4,9 and fluoxetine has been reported to cause false-positive results for LSD and amphetamines.1,3,9 Bupropion and trazodone have likewise been reported to cause false-positive LSD and amphetamine results, with the interaction to the amphetamine assay credited to cross-reactivity with the agents’ metabolites.1-4,9 Additionally, numerous reports have found venlafaxine to cause false-positive phencyclidine (PCP) results.2-4 While both venlafaxine and its active metabolite, O-desmethylvenlafaxine, are structurally dissimilar to PCP and have extremely low cross-reactivity (0.0125% and 0.025%, respectively), the concentrations of the two together have been hypothesized to cause the false-positive results.2,4
Furthermore, nearly all TCAs can cause false-positive UDS results. Amitriptyline, desipramine, doxepin, and imipramine have been reported to cause false-positive results for LSD,3 and desipramine and doxepin have additionally been reported to cause false-positive results for amphetamines.1,2 While rarely used, the monoamine oxidase inhibitor (MAOI) selegiline may also cause false-positive amphetamine results due to its l-amphetamine and l-methamphetamine metabolites.2,4
Minimizing the use of these agents in high-risk patients when possible may decrease the risk of false-positive results. For patients requiring an SSRI, pharmacists should consider using paroxetine, citalopram, or escitalopram and minimizing the use of fluoxetine and sertraline when appropriate. When using an antidepressant to treat neuropathic pain, minimizing the use of venlafaxine and TCAs and instead using duloxetine should be considered. Gabapentin and pregabalin have a minimal risk of causing false-positives and are other options that could be used. Trazodone is an antidepressant frequently used as a sleep aid. Minimizing its use and instead using mirtazapine or sedative-hypnotics when appropriate would be another consideration.1-4
Antipsychotics
In addition to antidepressants, many antipsychotic agents have also been reported to cause false-positive results. Antipsychotics may be used to treat a variety of psychiatric disorders, with the second-generation antipsychotics (SGAs) used more frequently due to their more favorable side-effect profile compared to the first-generation antipsychotics (FGAs). Of the SGAs, risperidone has been reported to cause false-positive LSD results;3,9 quetiapine, false-positive methadone and TCA results, which are attributed to quetiapine’s resemblance in structure to methadone and TCAs.2-4 Two case reports of accidental aripiprazole ingestion in pediatric patients resulted in false-positive amphetamine results.10 Whether false-positives with aripiprazole may also occur in adults is uncertain.10 The FGAs chlorpromazine, prochlorperazine, haloperidol, and thioridazine may all cause false-positive LSD results.3 Thioridazine may additionally cause false-positive amphetamine, methadone, and PCP results, and chlorpromazine cause false-positive amphetamine (due to similarities in structure) and methadone results.1-4
When selecting an antipsychotic agent for high-risk patients, consideration should be given to using lurasidone, olanzapine, or ziprasidone when appropriate. Aripiprazole may also be a reasonable option in adults, as no reports have found false-positive results in this population. However, pharmacists should carefully consider the possibility of a positive result being false should one occur with a patient on aripiprazole. Many of the FGAs cause false-positive UDS results and have a less favorable side-effect profile compared to the SGAs; thus, minimizing use of these agents when possible would be suggested.1-4
Other Central Nervous System (CNS) Medications
Other CNS agents that have been reported to cause false-positive UDS results include buspirone, carbamazepine, and lamotrigine (TABLE 2).1-4 Minimizing use of these agents when possible can also help reduce the risk of false-positive results.
Antiemetics
In addition to the antiemetics promethazine and doxylamine, metoclopramide and prochlorperazine have had documented false-positive LSD results.3 Consider minimizing the use of these agents and selecting 5-HT3 receptor antagonists such as ondansetron to decrease false readings in high-risk populations.3
Antibiotics
Most antibiotics have not been indicated to cause false-positives with UDS immunoassays; however, quinolones and rifampin have been documented in small studies.4 All quinolones have the potential to cause a false-positive opiate screening result, with levofloxacin and ofloxacin having the highest risk. Ciprofloxacin, moxifloxacin, and norfloxacin showed cross-reactivity to opiates because of similar molecular structures, but at lower levels than most immunoassay thresholds. Thus, these agents may be less likely to cause false-positives.4,11-12 Ofloxacin has also been reported to cause a false-positive amphetamine result.3 In addition to quinolones, rifampin has been shown to cause false-positives for opioids, and elimination calculations estimate a possible false-positive result for more than 18 hours after a single oral dose of rifampin 600 mg.13
OTCs
Determining what OTC products patients are taking is very important when using UDS testing, as some OTCs may cause false-positive results. Antihistamines, analgesics, cough suppressants, and heartburn medications have been shown to cause false-positives in studies and case reports.1-4
False-positive methadone levels have been documented with diphenhydramine 100 to 200 mg2-4,14 and doxylamine intoxication.4,15 Additionally, doxylamine intoxication has produced false-positive opiate14 and PCP2 levels, and brompheniramine use may cause false-positive amphetamine4 and LSD3,9 levels. Consider using second-generation antihistamines, as they have not been reported to cause false-positive UDS results.
Nonsteroidal anti-inflammatory drugs (NSAIDs) have also been shown to interact with UDS immunoassays. Both ibuprofen and naproxen have been documented to cause false-positive barbiturate4 and cannabinoid1-4 levels. In addition, ibuprofen can cause a false-positive PCP level.1-2,4 Consider minimizing the use of NSAIDs in high-risk patients and recommending acetaminophen instead.
The cough suppressant dextromethorphan may cause false-positive PCP1,2,4 and opioid levels due to its metabolite’s similarity to the opioid agonist levorphanol.1,2 Furthermore, decongestants phenylephrine and pseudoephedrine have shown false-positive amphetamine levels due to similar structures.1,2 To prevent misinterpretations, consider limiting these medications in high-risk populations.
Lastly, heartburn medications have been documented to interact with UDS tests to cause false-positives. Ranitidine has been shown to cause false-positive results for amphetamines at doses of 150 to 300 mg daily.16 On the other hand, pantoprazole has caused false cannabinoid results.1,2 Consider using other histamine blockers (e.g., famotidine) or proton pump inhibitors (e.g., omeprazole, esomeprazole, lansoprazole) not shown to cause false-positives.
Herbals
Herbal products may also interfere with UDS immunoassays. As morphine and codeine are derived from opium poppy seeds, the intake of relatively small amounts of poppy seeds may result in false-positives for opiates, including the consumption of poppy-seed cookies (having ~1 tsp of poppy-seed filling) or poppy-seed bagels.2 Additionally, the ingestion of foods containing hemp, such as hemp-seed oil, have resulted in positive marijuana UDS results,2 and ephedra-containing products may cause false-positive methamphetamine results.17
Other herbal supplements may be less likely to cause false-positive test results. A study of gingko biloba, saw palmetto, St. John’s wort, ginseng, garlic, green tea, valerian, and cranberry did not cause any false-positive reactions.18 Similarly, herbal teas and drinks did not cause any false-positives.19 Carefully assessing patient use of these products can help minimize misinterpretation of UDS results.
Conclusion
By recognizing common causes and medication concerns for false-negatives and false-positives in UDS testing, pharmacists can improve care and provide insight into alternative medications for patients. In all cases, clinical judgment should be used in selecting an appropriate therapeutic agent. Unexpected results from a UDS immunoassay should be checked with a confirmatory GC-MS or HPLC test. By reducing medication-related causes of false-positives and false-negatives, pharmacists can potentially decrease the need for additional testing and the negative consequences of misinterpreted urine immunoassay testing, thus optimizing patient care.
REFERENCES
1. Standridge JB, Adams SM, Zotos AP. Urine drug screening: a valuable office procedure. Am Fam Physician. 2010;81(5):635-640.
2. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76.
3. Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387-396.
4. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67(16):1344-1350.
5. Jaffee WB, Trucco E, Levy S, Weiss RD. Is this urine really negative? A systematic review of tampering methods in urine drug screening and testing. J Subst Abuse Treat. 2007;33(1):33-42.
6. Cone EJ, Lange R, Darwin WD. In vivo adul-teration: excess fluid ingestion causes false-negative marijuana and cocaine urine test results. J Anal Toxicol. 1998;22(6):460-473.
7. Johnson-Davis KL, Sadler AJ, Genzen JR. A retrospective analysis of urine drugs of abuse immunoassay true positive rates at a national reference library. J Anal Toxicol. 2016;40(2):97-107.
8. Breindahl T, Hindersson P. Methylphenidate is distinguished from amphetamine in drug-of-abuse testing. J Anal Toxicol. 2012:36(7):538-539.
9. Ritter D, Cortese CM, Edwards LC, et al. Interference with testing for lysergic acid diethylamide. Clin Chem. 1997;43:635-637.
10. Kaplan J, Shah P, Faley B, Siegel ME. Case reports of aripiprazole causing false-positive urine amphetamine drug screens in children. Pediatrics. 2015;136(6):e1625-e1628.
11. Baden LR, Horowitz G, Jacoby H, Eliopoulos GM. Quinolones and false-positive urine screening for opiates by immunoassay technology. JAMA. 2001;286(24):3115-3119.
12. Zacher JL, Givone DM. False-positive urine opiate screening associated with fluoroquinolone use. Ann Pharmacother. 2004;38(9):1525-1528.
13. de Paula M, Saiz LC, González-Revaldería J, et al. Rifampicin causes false-positive immunoassay results for urine opiates. Clin Chem Lab Med. 1998;36(4):241-243.
14. Kelner MJ. Positive diphenhydramine interference in the EMIT-d.a.u. assay. Clin Chem. 1984;30:1430.
15. Hausmann E, Kohl B, von Boehmer H, Wellhöner HH. False-positive EMIT indication of opiates and methadone in a doxylamine intoxication. J Clin Chem Clin Biochem. 1983;21(10):599-600.
16. Poklis A, Hall KV, Still J, et al. Ranitidine interfer-ence with the monoclonal EMIT d.a.u. amphetamine/methamphetamine immunoassay. J Anal Toxicol. 1991;15(2):101-103.
17. Markowitz JS, Donovan JL, DeVane CL, et al. Common herbal supplements did not produce false-positive results on urine drug screens analyzed by enzyme immunoassay. J Anal Toxicol. 2004;28:272-273.
18. Levisky JA, Karch SB, Bowerman DL, et al. False-positive RIA for methamphetamine following ingestion of an ephedra-derived herbal product. J Anal Toxicol. 2003;27(2):123-124.
19. Winek CL, Elzein EO, Wahba WW, et al. Interference of herbal drinks with urinalysis for drugs of abuse. J Anal Toxicol. 1993;17(4):246-247.
To comment on this article, contact rdavidson@uspharmacist.com.






