US Pharm. 2021;45(4):21-27.
ABSTRACT: At the end of 2019, a novel coronavirus now known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as the cause of a cluster of pneumonia cases in Wuhan, China. In February 2020, the World Health Organization named the outbreak coronavirus disease 2019 (COVID-19), and it became a global pandemic. By the end of 2020, several vaccines had become available for use in different parts of the world. In the United States, three vaccines have been granted emergency-use authorization (EUA) for the prevention of COVID-19. As frontline workers, pharmacists are critically needed both in educating patients on safety and efficacy of vaccines and in proper administration, counseling, and monitoring.
In the United States, as of April 2021, the COVID-19 mRNA vaccines BNT162b2 (Pfizer-BioNTech) and mRNA 1273 (Moderna) and the COVID-19 adenovirus vector vaccine Ad26.COV2.S (Janssen) have been granted emergency-use authorization (EUA) for prevention of COVID-19. In addition to the traditional process for issuing a license for a vaccine through FDA approval for an indication, the FDA can more rapidly make vaccines available in an emergency situation. This occurs through an EUA, which is designed to make products available during public-health emergencies. Under these circumstances, the FDA deems that the product “may be effective” and that the benefits are likely to outweigh the risks. The FDA has indicated that it will only issue an EUA for a SARS-CoV-2 vaccine if there is substantial evidence of safety and effectiveness, which includes meeting the prespecified efficacy criteria defined for the primary endpoint. A median of 2 months of follow-up for half of the vaccine participants after vaccine receipt is also required. As clinicians, pharmacists are required to inform potential recipients that these EUA vaccines are not licensed, why they are not licensed, and what information the FDA is waiting for before granting a full license. It is not necessary, however, for recipients to sign informed-consent documents.1-6
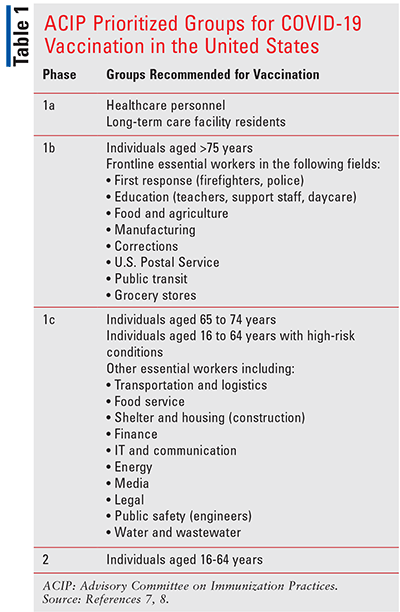
All three of these vaccines are being recommended for individuals who are eligible for vaccination according to local allocation priorities. BNT162b2 is indicated for persons aged 16 years or older and both mRNA 1273 and Ad26.COV2.S are indicated for individuals aged 18 years or older. The differences in age ranges included in the indications reflect the different age ranges included in the phase III trials. As initial vaccine supplies are limited, the Advisory Committee on Immunization Practices (ACIP) has recommended that these be allocated during the rollout in sequential phases (TABLE 1).7,8 The CDC has required that individual states draft plans for distributing and administering vaccines to these populations. Pharmacists should refer to their local public-health department for more detailed information, as some updates have been made depending on the state (e.g., many states were prioritizing vaccine eligibility to include those older than age 65 years). Pharmacists should address the differences in the magnitudes of effect reported from phase III trials that are related to factors other than effectiveness, including differences in the trial populations and locations, timing of the trials during the pandemic (because different variants arose later in the course of the pandemic), and study design. The choice between these vaccines is based on availability. They have not been compared directly, but they are all highly effective and have been shown to reduce the risk of COVID-19, especially severe/critical disease. Both the BNT162b2 and the mRNA 1273 vaccines use mRNA delivered in a lipid nanoparticle to express a full-length spike protein, while the Ad26.COV2.S is based on a replication-incompetent adenovirus 26 vector that expresses a stabilized spike protein.1-6
Clinical Trial Experience for EUA-Granted Vaccines
BNT162b2 (BioNTech-Pfizer): In a large placebo-controlled phase III trial, this vaccine had 95% efficacy (95% CI, 90.3-97.6) in preventing symptomatic COVID-19 at or after day 7 following the second dose. This effect was assessed after an analysis of 170 confirmed COVID-19 cases (eight in the vaccine group and 162 in the placebo group) among over 36,000 participants aged 16 years and older. Among adults aged 65 years and older who had other medical comorbidities or obesity, vaccine efficacy was 91.7% (95% CI, 44.2-99.8). Among the entire trial population, the rate of COVID-19 in the vaccine group started to decrease relative to the rate in the placebo group approximately 2 weeks after the first dose, suggesting some efficacy of a single dose (estimated vaccine efficacy of 52%; 95% CI, 29.5-68.4 between the two doses). However, the actual magnitude and duration of protection from a single dose is unknown because most participants received the second dose 3 weeks after the first.2,5,9-11
Local and systemic adverse effects were dose-dependent and relatively common after the second dose; most were of mild or moderate severity. The most common solicited adverse reactions were injection-site reactions (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), and fever (14.2%); severe adverse reactions occurred in 0.0% to 4.6% of participants, were more frequent after dose 2 than after dose 1, and were generally less frequent in adults aged 55 years and older (≤2.8%) as compared with younger participants (≤4.6%). Among adverse events of special interest, which could possibly be related to vaccine, lymphadenopathy was reported in 64 participants (0.3%): 54 (0.5%) in the younger (aged 16 to 55 years) group; 10 (0.1%) in the older (aged >55 years) group; and six in the placebo group. There were also rare reports of Bell’s palsy in the vaccine group and none in the placebo group, which calls for ongoing monitoring for possible vaccine-associated Bell’s palsy.2,5
mRNA 1273 (Moderna): In phase III results of a large, placebo-controlled trial, mRNA 1273 had 94.1% vaccine efficacy (95% CI, 89.3-96.8) in preventing symptomatic COVID-19 at or after 14 days following the second dose. This effect was assessed after an analysis of 196 confirmed COVID-19 cases (11 in the vaccine group and 185 in the placebo group) among nearly 30,000 study participants aged 18 years and older. Among adults aged 65 years and older, vaccine efficacy was 86.4% (95% CI, 61.4-95.5). In approximately 2,000 participants who received only a single dose of vaccine or placebo, vaccine efficacy following the dose was 80.2% (95% CI, 55.2-92.5); however, these individuals had a median follow-up time of only 28 days, so the duration of protection from a single dose remains uncertain.3,6,9-11
Local site reactions and systemic solicited events after vaccination were frequent and mostly mild to moderate. The most common solicited adverse reactions were injection site pain (91.6%), fatigue (68.5%), headache (63.0%), muscle pain (59.6%), joint pain (44.8%), and chills (43.4%); 0.2% to 9.7% were reported as severe, with severe solicited adverse reactions being more frequent after dose 2 than after dose 1 and generally less frequent in adults aged 65 years and older compared with younger participants. Lymphadenopathy was reported in 173 participants (1.14%) in the vaccine group and 95 participants (0.63%) in the placebo group. There was a numerical imbalance in hypersensitivity adverse events across study groups, with 1.5% of vaccine recipients and 1.1% of placebo recipients reporting such events in the safety set. Throughout the safety follow-up period to date, there have also been three reports of Bell’s palsy in the vaccine group and one in the placebo group. Currently, there is also ongoing monitoring for possible vaccine-associated Bell’s palsy for this vaccine.3,6
Ad26.COV2.S (Janssen): In a phase III efficacy trial detailed in briefing materials presented to the FDA, given as a single dose, the vaccine had 66.9% efficacy (95% CI, 59.0-73.4) in preventing moderate-to-severe/critical COVID-19 (which included patients with pneumonia, dyspnea, tachypnea, or at least two symptoms of COVID-19) starting at or after 14 days following vaccination. This effect was assessed after an analysis of 464 confirmed moderate-to-severe/critical COVID-19 cases (116 in the vaccine group and 348 in the placebo group) among nearly 40,000 study participants aged 18 years and older with a median follow-up of 2 months after vaccination. There were only four mild COVID-19 cases. Vaccine efficacy starting at 28 days after vaccination was similar to that after 14 days. Vaccine efficacy against severe/critical disease trended higher at 14 days postvaccination (78%) and 28 days postvaccination (85%). The reported overall efficacy varied by region: 74% in the U.S., 66% in Brazil, where the P.2 variant was prevalent, and 52% in South Africa, where most infections were caused by the variant B.1.351. Nevertheless, vaccine efficacy against severe/critical disease was similar across regions (and in South Africa was 72% and 83% after 14 and 28 days post-vaccination, respectively).4,12
Injection-site pain (49%), headache (39%), fatigue (38%), and myalgia (33%) were reported in vaccine recipients; fewer than 2% reported a grade 3 systemic side effect. Fever occurred following vaccination in 9%. These side effects were more common among those aged 59 years and younger compared with older participants. Overall, reactogenicity appeared less than with the mRNA COVID-19 vaccines. Serious adverse event rates in the vaccine and placebo group were similar. There were more cases of urticaria (5 vs. 1), thromboembolic events (15 vs. 10), and tinnitus (6 vs. 0) among vaccine recipients compared with placebo recipients.4,12
Dosing and Administration
BNT162b2 (Pfizer-BioNTech): BNT162b2 is administered in two IM doses of 0.3 mL each, given 3 weeks apart. If more than 21 days have elapsed after the first dose, the second dose can be given as soon as feasible without repeating the series. Each vial of BNT162b2 contains at least five doses after dilution. To maximize doses available and to address issues with low dead-space syringes, pharmacists should be aware of a revision made by the FDA to the package insert that the volume in each vial may be sufficient to supply six full doses. In such cases, all six doses can be administered, keeping in mind that any residual volume less than a full dose (i.e, <0.3 mL) should be discarded. This should also never be pooled with residuals from other vials.2
mRNA 1273 (Moderna): mRNA 1273 is administered in two IM doses of 0.5 mL each, given 1 month apart. If more than 28 days have elapsed after the first dose, the second dose can be given as soon as feasible without repeating the series.3
Ad26.COV2.S (Janssen): Ad26.COV2.S is administered IM as a single dose of 0.5 mL. As background information for pharmacists, note that it is also being evaluated as two doses 56 days apart.4
It is critical that pharmacists refer to the FDA Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers) for details and specific instructions on the storage and handling of the vaccine vials as these documents supersede the information on the carton and vial labels and differ from one vaccine to the other.2-4
Importantly, patients should complete the vaccine series for BNT162b2 and mRNA 1273 with the same vaccine initially used; there are no data to support the efficacy and safety of using one of the vaccines for the first dose and the other for the second. In the event that the same mRNA vaccine is temporarily unavailable for the patient, it is preferable to delay the second dose (up to 6 weeks) to receive the same product than to receive a mixed series with a different product. Also, other non–COVID-19 vaccines should not be administered within 14 days of COVID-19 vaccine administration as there are no data regarding safety and efficacy when these vaccines are coadministered with other vaccines.2,3,13
Special Populations
History of SARS-CoV-2 Infection: Individuals with recent, documented SARS-CoV-2 infection should have recovered from acute infection (if symptomatic) and met criteria for discontinuation of isolation precautions before receiving the vaccine. It is also recommended for such individuals to delay vaccination for 90 days from the time of infection to allow others to receive a vaccine sooner, as the risk of reinfection appears extremely low during this interval. The ACIP suggests that individuals who received monoclonal antibodies or convalescent plasma therapy for COVID-19 should delay vaccination for 90 days from the time of receipt.2-4,11
Pregnant and Breastfeeding Women: The safety of these vaccines has not yet been established in pregnant or breastfeeding women, and the fetal effects of the vaccines are unknown at this time. With those considering a COVID-19 vaccine, pharmacists should discuss information about the safety and efficacy of the vaccine, including information about the data that are not available, as this special subpopulation was not included in the clinical trials. The two vaccines that are mRNA vaccines do not contain a live virus but instead induce an immune response using viral mRNA. The theoretical risk of fetal harm from mRNA vaccines may be low, but we have no evidence for this. There is a pregnancy-exposure registry (v-safe) that monitors pregnancy outcomes in women exposed to the Ad26.COV2.S vaccine during pregnancy, and these women are encouraged to enroll as a means of collecting ongoing safety data for this population. The same statement regarding the lack of data would apply to breastfeeding. A conversation between the patient and the clinical team is critical for those considering inoculation in order to assist with their informed decision-making, weighing ongoing safety and the woman’s individual risk for severe disease.4,13,14
Children: The safety of these vaccines has not yet been established in children. Vaccine licensure will only include children once the safety and immunogenicity of the vaccine has been studied in them. Such studies are underway in older children and are planned in younger children.2-4
Immunocompromised Individuals: Key exclusion criteria in the trials included diagnosis with an immunocompromising condition. The immunogenicity and efficacy of the vaccines are uncertain in these populations, so it would be wise for these individuals to have a conversation about the uncertainties of the vaccine effect with their providers and weigh risks and benefits that can help them ultimately decide what is best for them.2-4
Patient Counseling
In addition to standard counseling about vaccine efficacy and safety, pharmacists are required to inform potential recipients that each COVID-19 vaccine is available under EUA and is not a licensed vaccine. Also, SARS-CoV-2 infection might still occur despite vaccination, and the duration of protection is uncertain; vaccine recipients should be reminded to continue other personal preventive measures to reduce SARS-CoV-2 transmission, because vaccines may not protect all vaccine recipients.2-4
Adverse Events
Pharmacists should counsel patients about experiencing possible local and systemic reactions (e.g., fever, chills, fatigue, headache) in the first 1 to 2 days after vaccination. Moreover, any respiratory symptoms would be atypical of a vaccine reaction, and these or systemic symptoms that occur after the first couple of days following vaccination should be immediately followed up with the primary-care provider.2-4
Adverse reactions following the BNT162b2 vaccine that have been reported in clinical trials include injection-site pain (84.1%), fatigue (62.9%), headache (55.1%), muscle pain (38.3%), chills (31.9%), joint pain (23.6%), fever (14.2%), injection-site swelling (10.5%), injection-site redness (9.5%), nausea (1.1%), malaise (0.5%), and lymphadenopathy (0.3%). Severe allergic reactions have been reported during mass vaccination outside of clinical trials.2
Adverse reactions reported in a clinical trial following administration of the mRNA 1273 vaccine include pain at the injection site (92%), fatigue (70%), headache (64.7%), myalgia (61.5%), arthralgia (46.4%), chills (45.4%), nausea/vomiting (23%), axillary swelling/tenderness (19.8%), fever (15.5%), swelling at the injection site (14.7%), and erythema (10%) at the injection site.3
The most common local, solicited adverse reaction (>10%) reported in the phase III ongoing trial following administration of the Ad26.COV2.S vaccine was injection-site pain (48.6%). The most common systemic adverse reactions (>10%) were headache (38.9%), fatigue (38.2%), myalgia (33.2%), and nausea (14.2%).4
Pharmacists should be aware that additional adverse reactions, some of which may be serious, may become apparent with more widespread use of the vaccines. Please refer to the Ongoing Safety Assessment section below on the requirement for pharmacists to report adverse reactions to these vaccines.3
Contraindications and Precautions
BNT162b2 and mRNA 1273 are each contraindicated in:
• Individuals with a severe allergic reaction, such as anaphylaxis, after a previous dose of an mRNA COVID-19 vaccine or to any of its components.2,3,13,14
• Individuals with an immediate allergic reaction of any severity (including hives) to a previous dose of an mRNA COVID-19 vaccine, to any of its components, or to polysorbate (with which there can be cross-reactive hypersensitivity to polyethylene glycol). Such individuals should not receive an mRNA COVID-19 vaccine unless they have been evaluated by an allergy expert who determines that it can be given safely.2,3,13,15
Ad26.COV2.S is contraindicated in4:
• Individuals with a known history of a severe allergic reaction, such as anaphylaxis, to any component of the vaccine.
Pharmacists may review the components of the mRNA and the Ad26.COV2.S COVID-19 vaccines listed on the CDC website. The ACIP lists a history of severe allergic reaction to any other vaccine or injectable therapy (that does not share the same components as the mRNA COVID vaccines) as a precaution to vaccination. Once patients are inoculated, pharmacists should monitor patients for 15 minutes after vaccination. Vaccines should be administered in settings where immediate allergic reactions, should they occur, can be appropriately managed. It is always advised that patients with a history of life-threatening reactions should speak with their physician first and should receive their vaccines under strict medical supervision and be monitored for 30 minutes postvaccination. This is because anaphylaxis has been reported following administration of both authorized mRNA COVID-19 vaccines. A proposed mechanism is the idea of it being IgE-mediated, with polyethylene glycol as the inciting antigen. However, the mechanism or mechanisms for these reactions are currently under investigation.2,3,12,15
Vaccines in Late-Stage Clinical Trial Development
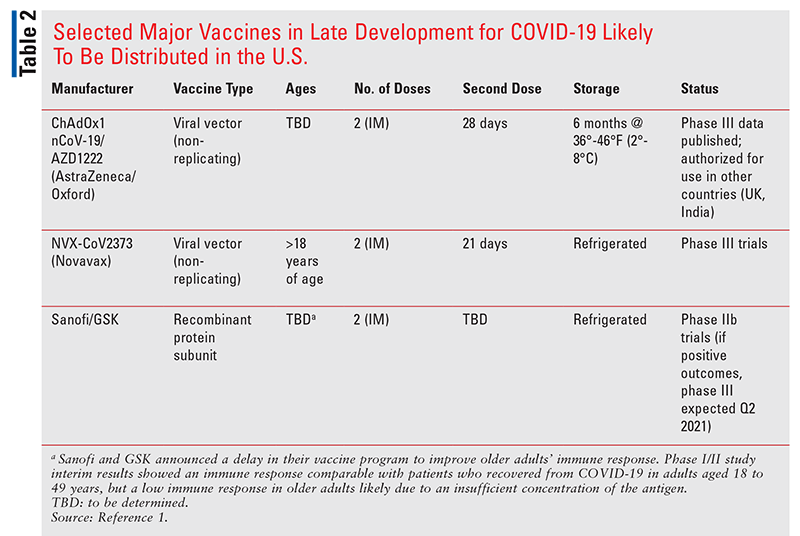
The first human clinical trials of SARS-CoV-2 vaccines began in March 2020, and several phase III trials are nearing completion. Selected vaccine candidates that have entered or are nearing entry into phase III trials and may be distributed in the U.S. are described in TABLE 2. They represent different vaccine approaches, including RNA vaccines, replication-incompetent vector vaccines, recombinant protein vaccines, and inactivated vaccines. For a complete list of vaccines in development, the World Health Organization maintains an updated list of vaccine candidates under evaluation.1
Postlicensure Monitoring
Although SARS-CoV-2 vaccines have received EUAs based on trials conducted in tens of thousands of participants, they are recommended for hundreds of millions of individuals. Thus, when used to this extent, efficacy questions that were not addressed in clinical trials will need to be monitored, and safety issues that were not initially evident may emerge.
There are still efficacy questions related to SARS-CoV-2 vaccination that the phase III trials have not answered. These include:
• Duration of protection from disease
• Potential need for and timing of additional booster doses
• Effectiveness in subpopulations not evaluated in the clinical trials
• Impact on community transmission (i.e., herd immunity)
These will require follow-up of trial participants, additional observational studies, and comprehensive surveillance systems.2-4,15
Ongoing Safety Assessment
Adequately assessing vaccine safety is critical to the success of immunization programs. Although existing comprehensive systems to monitor vaccine safety are in place, they are enhanced for the rollout of the SARS-CoV-2 vaccine program (TABLE 3).2-4,12,16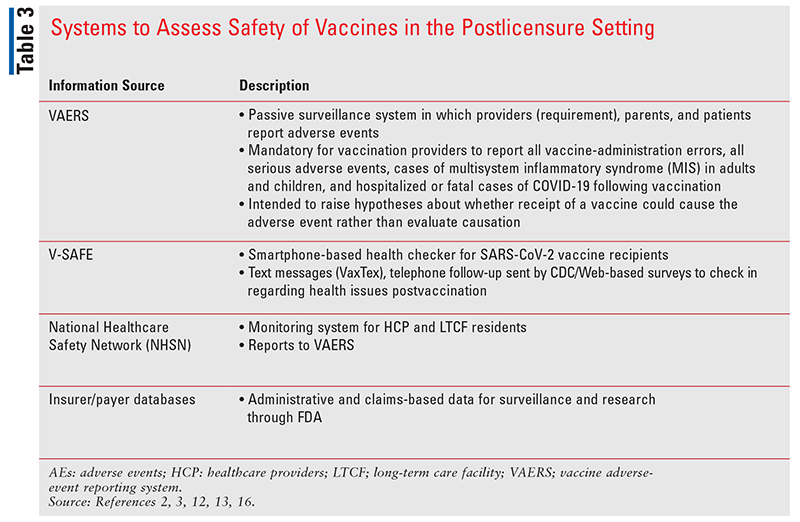
Conclusion
Based on evidence from other vaccines, pharmacists may improve vaccine acceptance in patients by identifying concerns, educating them on vaccine risks and benefits, and dispelling misconceptions about the disease and the vaccine. Pharmacists should always respect the patient’s informed decision on whether to vaccinate or not and should be empathetic and understanding of each person’s views and decision. Although some of these data from phase III trials are hopeful, full trial reports are still needed to critically assess the vaccines’ impact and safety, including effects on microbiologically confirmed SARS-CoV-2 infection (both asymptomatic and symptomatic, which could have implications for transmission). The durability of effect, as well as long-term safety, will also need to be evaluated over time using both real-world data and the monitoring that will continue for 2 years after administration of the second dose of the vaccines from the clinical trials.
Note: On April 13th, the CDC and FDA recommended a temporary pause in the use of the Johnson & Johnson (Janssen) COVID-19 vaccine out of an abundance of caution. Of over 6.8 million doses administered nationally, there have been six reported cases of a rare and severe type of blood clot called cerebral venous sinus thrombosis seen in combination with low levels of blood platelets (thrombocytopenia), with symptoms occurring 6 to 13 days after vaccination. All of these reported cases occurred among women between the ages of 18 and 48 years. Treatment of this specific type of blood clot is different from the treatment that might typically be administered. Usually, the anticoagulant drug heparin is used to treat blood clots. In this setting, administration of heparin may be dangerous, and alternative treatments need to be given. The CDC convened a meeting of the Advisory Committee on Immunization Practices on April 14th to further review these cases and assess their potential significance. The committee decided to meet again in 1 to 2 weeks for further discussion and did not change the current “paused” status of the vaccine as it is still analyzing incoming safety reports Pharmacists should be made aware that those people who have received the Janssen vaccine who develop severe headache, abdominal pain, leg pain, or shortness of breath within 3 weeks after vaccination should contact their healthcare provider. As standard practice, pharmacists are asked to report adverse events to the Vaccine Adverse Event Reporting System at https://vaers.hhs.gov/reportevent.html.17
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines. www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Accessed January 12, 2021.
2. FDA. Emergency Use Authorization (EUA) of the Pfizer-BioNTech COVID-19 vaccine to prevent COVID-19. Revised February 25, 2021. Fact sheet for healthcare providers administering vaccine. www.fda.gov/media/144413/download. Accessed March 29, 2021.
3. FDA. Emergency Use Authorization (EUA) of the Moderna COVID-19 vaccine to prevent COVID-19. Fact sheet for healthcare providers administering vaccine. Revised December 2020. www.fda.gov/media/144637/download?utm_medium=email&utm_source=govdelivery. Accessed January 10, 2021.
4. FDA. Emergency Use Authorization (EUA) of the Janssen COVID-19 vaccine to prevent COVID-19. Factsheet for healthcare providers administering vaccine. Revised February 27, 2021. www.fda.gov/media/146304/download. Accessed March 5, 2021.
5. FDA. Vaccines and Related Biological Products Advisory Committee meeting December 10, 2020. FDA briefing document: Pfizer-BioNTech COVID-19 vaccine. www.fda.gov/media/144245/download. Accessed March 5, 2021.
6. FDA. Vaccines and Related Biological Products Advisory Committee meeting December 17, 2020. FDA briefing document: Moderna COVID-19 vaccine. www.fda.gov/media/144434/download. Accessed March 5, 2021.
7. Dooling K, McClung N, Chamberland M, et al. The Advisory Committee on Immunization Practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(49):1857-1859.
8. ACIP COVID-19 Vaccine Working Group. Phased allocation of
COVID-19 vaccines. December 20, 2020. www.cdc.gov/vaccines/acip/
meetings/downloads/slides-2020-12/slides-12-20/02-COVID-Dooling-508.pdf. Accessed March 16, 2021.
9. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383:2603-2615.
10. Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2020; 384(1):80-82.
11. Walsh EE, Frenck RW Jr, Falsey AR, et. al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439-2450.
12. FDA. FDA briefing document. Janssen Ad26.COV2.S vaccine for the prevention of COVID-19. Vaccines and Related Biological Products Advisory Committee meeting, February 26, 2021. www.fda.gov/media/146217/download. Accessed March 5, 2021.
13. CDC. Clinical considerations for use of COVID-19 vaccines currently authorized in the U.S. Revised March 5, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html. Accessed March 29, 2021.
14. American College of Obstetricians and Gynecologists’ (ACOG) Immunization, Infectious Disease, and Public Health Preparedness Expert Work Group in collaboration with Riley LE, et al. Vaccinating pregnant and lactating patients against COVID-19. Updated March 24, 2021. www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19. Accessed March 29, 2021.
15. CDC. Interim clinical considerations for use of COVID-19 vaccines currently authorized in the United States. Updated March 5, 2021. www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html. Accessed March 29, 2021.
16. CDC. What to do if you have a severe allergic reaction after getting a COVID-19 vaccine. Updated March 4, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/allergic-reaction.html. Accessed March 29, 2021.
17. CDC. Joint CDC and FDA Statement on Johnson & Johnson COVID-19 Vaccine. www.cdc.gov/media/releases/2021/s0413-JJ-vaccine.html. Accessed April 16, 2021.
To comment on this article, contact rdavidson@uspharmacist.com.






