US Pharm. 2010;35(1):46-48.
Vascular dementia (VaD) is impairment of memory and cognitive functioning that is caused by cerebrovascular disease. VaD is widely accepted as the second most common cause of cognitive impairment; Alzheimer's disease (AD) is the first. Despite the high prevalence of VaD, the chemical and biologic mechanisms are poorly understood and no specific pathologic criteria for diagnosis exist.1,2
Historically, patients with dementia due to recurrent strokes were diagnosed with multi-infarct dementia; the currently accepted term is VaD, since the disease can occur after a single vascular incident.2,3 However, both of these terms lack emphasis on early detection. In the future, the preferred appellation will likely be vascular cognitive impairment (VCI), an umbrella term including multi-infarct dementia or VaD and mild cognitive impairment due to vascular causes. VCI stresses the importance of recognizing the disease sooner, since VaD may be somewhat avoidable through risk-factor modification and stroke prevention.2 This article will address risk-factor management and treatment options for VaD.
Epidemiology
It is estimated that 6% to 10% of people over the age of 65 have dementia, and the percentage escalates with increasing age.4 Although AD is the predominant form in the United States, affecting nearly 5 million people, up to 60% of people with AD likely have an overlap with VaD. VaD alone is presumed to account for nearly 30% of dementia cases in North America and Europe. More than 1 million elderly people in the U.S. are affected by VaD, with most cases undiagnosed.5 Epidemiologic studies indicate higher rates of VaD in men, people of Asian descent, and older people.6
According to the CDC, 795,000 strokes occur each year in the U.S., and approximately 25% of them are in individuals with a prior stroke.7 Furthermore, 75% of all strokes occur in individuals older than 65 years.5 In poststroke patients, age is the greatest risk factor for the development of VaD.7
Despite these epidemiologic findings and projections, accurately determining the incidence of VaD remains difficult owing to differences in screening methods and diagnostic criteria. Cognitive status after a stroke is unstable, and there is wide variability in rates of cognitive impairment and dementia after stroke, regardless of study duration.1
Etiology
There are four common pathologies that contribute to VaD: large-artery infarctions, small-artery infarctions, recurrent infarctions, and hypoperfusion. Intracerebral hemorrhage may contribute to VaD, but is not generally described as a pathology.1
Large-artery infarctions are responsible for most poststroke dementia cases. Research has established a strong relationship between age, low education level, and poststroke dementia. A correlation between poststroke dementia and vascular risk factors (e.g., hypertension, diabetes, hyperlipidemia, smoking) also has been made. It is unknown whether the correlation is compounded in patients with multiple risk factors.2
Small-artery infarctions and hypoperfusion are mostly responsible for mild cognitive impairment. These pathologies lead to white-matter lesions (WML), which can be seen on MRI.2 WML are a nonspecific radiologic finding that can be caused by vascular and nonvascular pathologies. The Framingham study demonstrated an association between WML and declines in executive function, new learning, and visual organization.2 WML have been associated with age, hypertension, chronic kidney disease, metabolic syndrome, microvascular retinopathy, elevated homocysteine levels, low vitamin B12 levels, and elevated C-reactive protein levels.1
Diagnosis
There are no uniform diagnostic criteria for VaD. The four most common clinical references used are the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; the State of California Alzheimer's Disease Diagnostic and Treatment Centers (ADDTC) criteria; the National Institute of Neurological Disorders and Stroke and Association Internationale pour la Recherché et l'Enseignement en Neurosciences (NINDS-AIREN) criteria; and the International Statistical Classification of Diseases, 10th revision.
Except for ADDTC, the diagnostic criteria require a memory disorder in addition to other cognitive impairment. ADDTC and NINDS-AIREN require neuroimaging studies indicating cerebrovascular lesions, as well as a temporal relationship between cognitive or functional decline with the stroke event. NINDS-AIREN is the most widely used criterion, with patients classified as definite, probable, or possible with regard to a diagnosis of VaD. The various diagnostic criteria do not identify the same patients; this accounts for the variations in published prevalence and incidence rates and in treatment outcomes.1
Because the pathogenesis, risk factors, and comorbidities for VaD are similar to those for AD, it is sometimes difficult to differentiate the two.3 VaD predominantly involves deficits in cognitive functioning, whereas AD involves earlier and more significant memory impairment (TABLE 1).5 The Hachinski Ischemic Score (HIS) is a diagnostic tool used to differentiate VaD and AD.1 Since CT and MRI were not available at the time of its development in 1974, the HIS does not take neuroimaging into account.3 The HIS remains in use since no other diagnostic tool has been validated to differentiate VaD and AD.
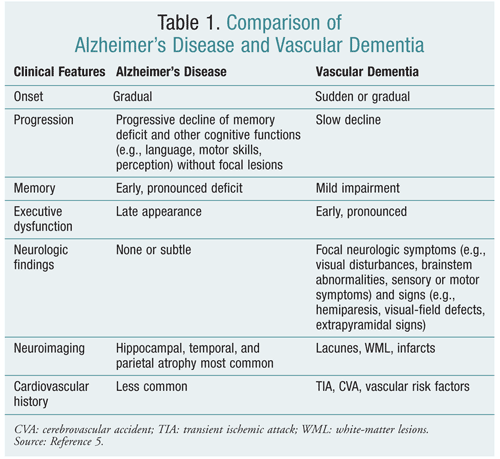
Although neuroimaging is not required for diagnosis by all clinical references, it is recommended in patients with suspected VaD due to stroke history, vascular risk factors, or abnormal neurologic examination. If CT shows significant evidence of vascular pathology, MRI is not necessary.1 However, MRI is more sensitive than CT for ischemic damage and vascular lesions.2 The absence of cerebral vascular lesions on CT or MRI excludes the diagnosis of VaD. Features on CT or MRI that are suggestive of VaD include cortical or subcortical infarctions, multiple lacunar strokes, and WML.8
Treatment and Prevention
There are no FDA-approved drugs for the treatment of VaD. However, the use of cholinesterase inhibitors and N-methyl-d-aspartate receptor antagonists for VaD has been evaluated. Donepezil (Aricept) was the first cholinesterase inhibitor studied for VaD. Two large (>300 subjects each), randomized, placebo-controlled trials of donepezil 5 mg to 10 mg per day showed improvement in AD Assessment Scale-Cognitive (ADAS-Cog) subscale scores. ADAS-Cog, the most popular instrument in clinical trials of cholinesterase inhibitors, measures deficits in memory, language, executive functioning, attention, and other cognitive abilities. A long-term extension showed continued benefit at 54 weeks, with greater improvement in subjects who started the drug at baseline than in those who started in the extension arm.9-11 In March 2006, Eisai--the manufacturer of donepezil--terminated a phase III trial evaluating the use of donepezil in VaD because of an increased risk of death in patients receiving active drug.12
Galantamine (Razadyne) has been studied for the treatment of VaD in two randomized, double-blind, placebo-controlled trials of 592 patients and 788 patients, respectively. ADAS-Cog score was a primary efficacy measure. At 26 weeks, there were improvements in galantamine-treated patients at doses of 16 mg to 24 mg per day. However, a significant percentage of patients dropped out owing to adverse events, particularly gastrointestinal effects.2,13,14
Rivastigmine (Exelon) was studied in a randomized, placebo-controlled trial of 710 patients aged 50 to 85 years. ADAS-Cog score was a primary efficacy measure. Rivastigmine was superior to placebo, except in younger (age 50-75 years) patients. It was speculated that younger patients were more likely to have VaD-related pathology and older patients had more AD-type pathology. Younger patients experienced increased blood pressure, more cerebrovascular accidents, and increased mortality versus older patients.15
Memantine (Namenda) was studied for the treatment of VaD in two large trials (321 patients and 579 patients, respectively). These randomized, double-blind, placebo-controlled studies measured ADAS-Cog scores. Both trials concluded that memantine 20 mg per day improved ADAS-Cog scores, but no other scales were used.16,17
Importantly, the scales used to measure clinical significance in these trials were developed for AD.18 It is also hard to draw conclusions about which patients are most likely to benefit from these drugs. Underlying pathology and age may predict response, but more research is needed. Current data do not warrant the widespread use of cholinesterase inhibitors or memantine for VaD; for now, clinical judgment must guide individual treatment decisions. Trials of cholinesterase inhibitors for VaD are ongoing or actively recruiting; see ClinicalTrials.gov.19
Once VaD or VCI has been diagnosed, attention should be directed at prevention and modification of risk factors. Research is exploring a possible relationship between hypertension, high serum cholesterol, and diabetes in the development of VaD. Studies are examining the effect of antihypertensive therapies--including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and calcium channel blockers--on the development of dementia and cognitive decline. In general, reductions in blood pressure decrease the incidence of dementia. It is not clear whether the benefit of antihypertensive therapy is due solely to lowering of blood pressure or whether these drugs have other neuroprotective effects.2
Macrovascular complications of diabetes include heart disease and stroke; there also is an association between diabetes and dementia. An extension of the Action to Control Cardiovascular Risk in Diabetes trial is currently examining the effects of glycemic control on cognitive function. It is theorized that controlling blood glucose may decrease the risk of VCI.2 Importantly, hypoglycemia can impair cognitive function in elderly patients. Blood-glucose control and hypoglycemia avoidance may be a difficult balance to achieve in the geriatric population.
Because lipid-lowering agents are key in preventing cardiovascular disease, they are of interest for prevention of VaD. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels study found a 16% reduction in recurrent stroke in patients with a history of stroke or transient ischemic attacks.2 Other trials have shown positive and negative effects on dementia. It is hoped that lowering cholesterol reduces VCI risk.
Aspirin and other antiplatelet therapies are effective for stroke prevention. Pentoxifylline and aspirin are somewhat beneficial in multi-infarct dementia.20,21 Patients are being recruited to study the use of cilostazol versus aspirin for VaD.19 Antiplatelet agents generally have not been studied for treatment of VCI. The American Heart Association/American Stroke Association and American College of Chest Physicians' recommendations for secondary prevention of stroke should be followed until more data on antiplatelet agents for treating VaD are available. Aspirin 50 mg to 325 mg per day, clopidogrel 75 mg per day, and aspirin 25 mg/extended-release dipyridamole 200 mg twice per day are acceptable treatments.22,23
Pharmacist's Role
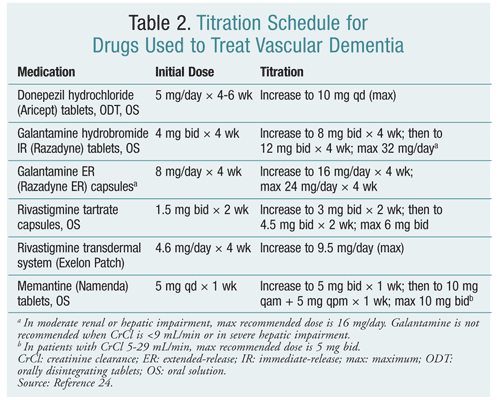
By 2030, people aged 65 years or more will constitute nearly 20% of the population.4 As advances in prevention and treatment improve survival rates, VaD and VCI incidence is expected to rise. It will be increasingly important for pharmacists to understand the prevention and treatment of VaD. Cholinesterase inhibitors and memantine may be options for some patients; their use should be judicious, with attention paid to achieving therapeutic doses according to manufacturers' schedules (TABLE 2).24 Owing to limited clinical evidence, side-effect profiles, and cost, continued use of these drugs should be confined to patients who exhibit improved cognitive functioning. Until further research is conducted, pharmacists should focus on improving the treatment of hypertension, hyperlipidemia, and diabetes and preventing primary and secondary stroke.
REFERENCES
1. Wright CB. Etiology, clinical manifestations, and diagnosis of vascular dementia. Waltham, MA: UpToDate Inc; June 2009. http://uptodateonline.com/
2. Rojas-Fernandez CH, Moorhouse P. Current concepts in vascular cognitive impairment and pharmacotherapeutic implications. Ann Pharmacother. 2009;43:1310-1323.
3. Knopman DS. Dementia and cerebrovascular disease. Mayo Clin Proc. 2006;81:223-230.
4. Chapman DP, Williams SM, Strine TW, et al. Dementia and its implications for public health. Prev Chronic Dis. 2006;3:A34.
5. Pinkston JB, Alekseeva N, Gonzàlez-Toledo E. Stroke and dementia. Neurol Res. 2009;31:824-831.
6. Fratiglioni L, Rocca WA. Epidemiology of dementia. In: Boller F, Cappa S, eds. Handbook of Neuropsychology: Vol. 6: Aging and Dementia. 2nd ed. Amsterdam, Netherlands: Elsevier Science; 2001.
7. Centers for Disease Control and Prevention. Stroke facts and statistics. www.cdc.gov/Stroke/stroke_
8. Farlow MR. Use of antidementia agents in vascular dementia: beyond Alzheimer disease. Mayo Clin Proc. 2006;81:1350-1358.
9. Wilkinson D, Doody R, Helme R, et al. Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology. 2003;61:497-486.
10. Black S, Román GC, Gelmacher DS, et al. Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke. 2003;34:2323-2330.
11. Wilkinson D, Róman G, Salloway S, et al. The long-term efficacy and tolerability of donepezil in patients with vascular dementia. Int J Geriatr Psychiatry. July 21, 2009:Epub.
12. Alzheimer's Association. Vascular dementia. www.alz.org/alzheimers_
13. Auchus AP, Brashear HR, Salloway S, et al. Galantamine treatment of vascular dementia: a randomized trial. Neurology. 2007;69:448-458.
14. Craig D, Birks J. Galantamine for vascular cognitive impairment. Cochrane Database Syst Rev. 2006;(1):CD004746.
15. Ballard C, Sauter M, Scheltens P, et al. Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: the VantagE study. Curr Med Res Opin. 2008;24:2561-2574.
16. Orgogozo JM, Rigaud AS, Stoffler A, et al. Efficacy and safety of memantine in patients with mild to moderate vascular dementia; a randomized, placebo-controlled trial (MMM300). Stroke. 2002;33:1834-1839.
17. Wilcock G, Möbius HJ, Stöffler A. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500). Int Clin Psychopharmacol. 2002;17:297-305.
18. Molnar FJ, Man-Son-Hing M, Fergusson D. Systematic review of measures of clinical significance employed in randomized controlled trials of drugs for dementia. J Am Geriatr Soc. 2009;57:536-546.
19. Clinical Trials.gov. List results: vascular dementia. www.clinicaltrials.gov/ct2/
20. Meyer JS, Rogers RL, McClintic K, et al. Randomized clinical trial of daily aspirin therapy in multi-infarct dementia. A pilot study. J Am Geriatr Soc.1989;37:549-555.
21. European pentoxifylline multi-infarct dementia study. Eur Neurol. 1996;36:315-321.
22. Adams RJ, Albers G, Alberts MJ, et al. Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2008;39:1647-1652.
23. Albers GW, Amarenco P, Easton D, et al. Antithrombotic and thrombolytic therapy for ischemic stroke: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133(suppl 6):630S-669S.
24. Micromedex Healthcare Series [by subscription]. www.thomsonhc.com.ezproxy.
This material is the result of work supported with resources and the use of facilities at the Bay Pines VA Healthcare System. This article does not represent the views of the Department of Veterans Affairs or the U.S. government.
To comment on this article, contact rdavidson@uspharmacist.com.





