US Pharm. 2013;38(3)(Oncology suppl):8-11.
ABSTRACT: Cancer patients are at considerable risk for venous thromboembolism (VTE). Risk stratification is essential to ensure that cancer patients at higher risk receive appropriate prophylaxis. Factors that increase VTE risk include obesity, advanced age, and other comorbidities. Patients with hematologic malignancies such as multiple myeloma have the greatest risk of VTE. Furthermore, drugs used to treat multiple myeloma (e.g., thalidomide, lenalidomide) are associated with increased risk of VTE and carry black box warnings. Current guidelines recommend using a risk-assessment tool to stratify patients with multiple myeloma. Patients with multiple risk factors appear more likely to benefit from low-molecular-weight heparin or warfarin, but it remains unclear whether initiation of anticoagulation therapy improves mortality and outweighs the risk of bleeding. New oral anticoagulants with fixed dosing may offer an advantage over conventional therapies in this population, but supportive data are lacking. Pharmacists can play an integral role in educating patients and providers about the risk of VTE associated with multiple myeloma treatment and in identifying high-risk patients who may benefit from anticoagulation therapy.
Patients with cancer are at significant risk for thrombosis, a major complication and a potentially life-threatening condition.1 Venous thromboembolism (VTE), which includes deep venous thrombosis and pulmonary embolism, affects up to 20% of cancer patients.1 Additionally, cancer patients with an acute VTE episode have an eightfold higher risk of mortality compared with patients without cancer.2 Several pathophysiological factors increase VTE risk, including a hypercoagulable state due to cancer cell procoagulant production, vessel-wall damage, and vessel stasis from direct compression.3 The risk of VTE in the cancer patient is influenced by patient-related factors (advanced age, obesity, comorbidities [e.g., infection], immobility), the cancer site and type, and the treatment used. Cancers associated with the highest VTE rates include malignancies of the pancreas, kidney, ovary, lung, and stomach.1
Patients with hematologic malignancies such as multiple myeloma also have high rates of VTE.4 VTE incidence in patients with multiple myeloma varies widely, but is estimated to be 5% to 10%.5 Various factors are thought to influence the development of VTE in multiple myeloma patients, including postsurgical immobility, the presence of central venous catheters, preexisting or acquired abnormalities in clotting-factor production or platelet function, and treatment with angiogenesis inhibitors (e.g., thalidomide, lenalidomide).5 The purpose of this article is to review current prophylaxis options and the potential role of newer anticoagulants for VTE prophylaxis in ambulatory cancer patients with multiple myeloma.
Considerations in Multiple Myeloma Patients
Thalidomide and lenalidomide are widely used in the management of multiple myeloma, often in combination with cytotoxic chemotherapy and/or dexamethasone.5 Unfortunately, these agents have been linked to venous and arterial thrombotic events when used in these combinations.5 It has been postulated that the increased VTE risk is due to reduced levels of thrombomodulin, a receptor responsible for binding to thrombin and converting it from a procoagulant enzyme to an anticoagulant enzyme.6 It also has been hypothesized that thalidomide and lenalidomide restore the expression of protease-activated receptor 1 (PAR1) in patients treated with doxorubicin, which reduces expression of PAR1.7 When the PAR1 receptor is expressed on the endothelial cell, thrombin is allowed to bind and to exert its proatherogenic effects. This may explain the hypercoagulable state occurring in patients treated concomitantly with cytotoxic agents such as doxorubicin. Additional VTE risk has been observed in patients also receiving dexamethasone, which is believed to damage the endothelium, thereby predisposing it to clotting.8 Thus, both thalidomide and lenalidomide have a black box warning for increasing VTE risk.9,10
As previously noted, cancer patients with acute VTE have a high mortality rate, but anticoagulant prophylaxis may decrease mortality. A systematic review of 11 randomized, controlled trials found improved survival rates at 1 year when anticoagulants were used, particularly low-molecular-weight heparin (LMWH), but anticoagulant use was associated with an increase in bleeding risk.11 It is theorized that anticoagulants may improve survival in certain tumor types and stages.11 Data are limited, however, so the widespread use of anticoagulant prophylaxis to improve survival is not currently recommended by consensus guidelines.1 Further investigations are under way to elucidate the role of anticoagulant prophylaxis in improving cancer mortality.
Despite the high incidence of VTE in the cancer population and the potential mortality benefit of anticoagulant therapy, the risk of bleeding must be considered before anticoagulant prophylaxis is initiated, since cancer patients on warfarin have a higher bleeding risk than noncancer patients, regardless of international normalized ratio (INR).1 This suggests that other factors, such as thrombocytopenia development and tumor penetration of organs, may contribute to the increase in overall bleeding risk in cancer patients. Warfarin may be associated with an absolute increase in the risk of major and minor bleeding, whereas LMWH and unfractionated heparin (UFH) are less detrimental in this regard.12 To prevent VTE in ambulatory cancer patients, the risk of bleeding must be carefully considered on an individual basis before anticoagulant prophylaxis is initiated.
Guidelines and Risk Assessment
The American Society of Clinical Oncology and the National Comprehensive Cancer Network (NCCN) guidelines neither recommend nor discourage VTE prophylaxis in ambulatory cancer patients.1,13 However, the American College of Chest Physicians (ACCP) Evidence-Based Clinical Practice Guidelines appear to favor LMWH or UFH over warfarin, but suggest LMWH or UFH prophylaxis only in cancer patients with risk factors such as history of VTE, immobility, hormonal therapy, and use of angiogenesis inhibitors (e.g., thalidomide and lenalidomide).12 The paucity of data limits the ability of these consensus groups to provide a concise recommendation regarding when and how to prevent VTE. Evidence appears strongest for multiple myeloma patients who are receiving thalidomide- or lenalidomide-based combination regimens, particularly when thalidomide or lenalidomide is combined with dexamethasone or doxorubicin, or in the case of multiagent chemotherapy regimens.1
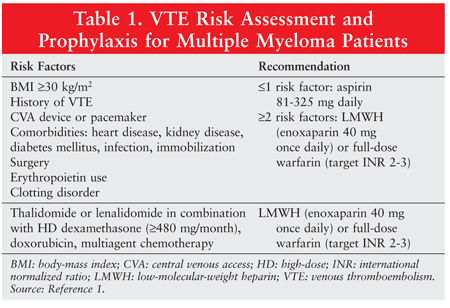
Few reliable models exist to assist clinicians in deciding whether to initiate anticoagulant therapy in high-risk cancer patients. NCCN guidelines provide a risk-assessment tool (TABLE 1) that may be used to determine whether VTE prophylaxis is warranted in ambulatory cancer patients with multiple myeloma.1 Multiple myeloma patients with one or no risk factors may be given aspirin 81 mg to 325 mg once daily. If two or more risk factors are present or if the patient is receiving angiogenesis agents in combination with high-dose dexamethasone, doxorubicin, or chemotherapy, clinicians should consider using LWMH (e.g., enoxaparin 40 mg daily) or full-dose warfarin (target INR 2-3).1 These recommendations do not include UFH as an option, but UFH would be a reasonable alternative to LMWH.
VTE Prophylaxis Options
Aspirin: The ACCP recommends against the use of aspirin for VTE prevention in any setting.12 In the multiple myeloma population, however, aspirin has been studied as an option because many patients are already taking it for other indications, it requires no monitoring, it is easily accessible, and it is inexpensive. Additionally, thalidomide has been shown to cause platelet aggregation, which can be abrogated with aspirin. The NCCN guidelines recommend aspirin 81 mg to 325 mg daily as an option only for multiple myeloma patients with one or no risk factors.1 Data suggest that aspirin is at least as effective as warfarin, compared with LMWH.14 Furthermore, aspirin carries a lower risk of major hemorrhagic complications than do anticoagulants. Potential concerns regarding aspirin use include the possibility of aspirin resistance and uncertainty about optimal dosing. Nevertheless, aspirin remains a reasonable prophylaxis option in low-risk multiple myeloma patients.
Vitamin K Antagonist: Warfarin is an oral anticoagulant commonly used for VTE prevention and treatment in a variety of patient populations. Fixed-dose warfarin (1-2 mg/day) and full-intensity warfarin (target INR 2-3) are two approaches that have been investigated.5 Studies evaluating the use of fixed-dose warfarin have yielded mixed results, whereas the full-intensity approach appears to confer a higher risk of bleeding complications.5 Limitations to warfarin use are numerous, including the need for regular INR monitoring, and the effectiveness of warfarin is affected by drug-drug interactions, kidney and liver function status, dietary intake of vitamin K, and alcohol. The constant need to monitor INR places an additional burden on the patient, and anticoagulation clinics are not readily accessible to all patients. Supratherapeutic INRs can be problematic owing to an increased risk of bleeding complications. Additionally, patients experiencing chemotherapy-induced nausea and vomiting may be unable to swallow the warfarin tablet, resulting in decreased absorption and further variability in INR. Despite these barriers, NCCN guidelines recommend full-intensity warfarin as an option in multiple myeloma patients with two or more risk factors because of its success in preventing VTE in other indications.1
Parenteral Anticoagulants: Fixed-dose UFH, enoxaparin, and fondaparinux are parenteral anticoagulants that may be used for VTE prophylaxis.1 These subcutaneous injections may be given in inpatient and outpatient settings and may be more easily administered in patients unable to tolerate oral medications because of chemotherapy-induced nausea and vomiting. More important, routine anticoagulation monitoring generally is not required, except in patients with renal impairment or other disease-related conditions. Fixed, weight-adjusted dosing is also more convenient.
Subcutaneous UFH may be administered as 5,000 U three times daily.1 Although the ACCP guidelines list it as an option for VTE prophylaxis, UFH is not identified specifically for the cancer population.12 Enoxaparin is a commonly used alternative to UFH since it is dosed once daily, and several randomized, controlled trials support the use of LMWH over warfarin in ambulatory cancer patients owing to its greater effectiveness and lower risk of bleeding.13 Fondaparinux 2.5 mg once daily is less readily used because of concern regarding its long half-life, and it is contraindicated in patients with creatinine clearance (CrCl) less than 30 mL/min.1 Data on and experience with fondaparinux in cancer patients are limited, but the agent is a viable option in patients who develop thrombocytopenia while taking UFH or enoxaparin.1 The NCCN guidelines specifically recommend enoxaparin 40 mg once daily for VTE prophylaxis in multiple myeloma patients taking antiangiogenesis agents.1 For patients with severe renal insufficiency (CrCl <30 mL/min), the enoxaparin dosage should be reduced to 30 mg once daily. Enoxaparin and fondaparinux are not as easily reversed as warfarin, and they should be carefully considered in patients at high risk for bleeding.1
Potential of New Anticoagulants: The limitations of warfarin and parenteral anticoagulants have led to much interest in oral fixed-dose anticoagulants.3 These agents include dabigatran, rivaroxaban, and apixaban, which are FDA approved for stroke prevention in patients with atrial fibrillation. Rivaroxaban, however, is the only one with an additional indication for VTE treatment and prevention of VTE occurrence. Currently, it is not approved for primary prophylaxis of VTE. Rivaroxaban dosing for VTE treatment is 15 mg twice daily for 21 days, after which the dose is reduced to 20 mg once daily. The use of dabigatran, rivaroxaban, and apixaban in patients with renal impairment requires a dosing adjustment since elimination occurs primarily through the kidneys, which could be a concern in cancer patients receiving nephrotoxic chemotherapy. As with warfarin, these agents are not devoid of drug-drug interactions. Rivaroxaban and apixaban are metabolized via CYP3A4 pathways, while all of these newer agents interact with potent P-glycoprotein inhibitors (e.g., quinidine) and inducers (e.g., rifampin), which may increase or decrease anticoagulant effectiveness, respectively. As with enoxaparin and fondaparinux, reversal of these newer anticoagulants during an acute bleeding episode is a concern, as no antidote currently exists. Data on cancer patients are not available, but these new anticoagulants seem promising.3
Nonpharmacologic Prophylaxis Options
Mechanical prophylaxis options include intermittent pneumatic compression (IPC) and graduated compression stockings (GCS), which are commonly used in patients with contraindications to pharmacologic therapies or in addition to anticoagulants in high-risk patients.1 IPC devices are used in the inpatient setting for postsurgical patients. GCS are an option for ambulatory cancer patients. One major drawback of GCS is that supportive data regarding their effectiveness in preventing VTE are limited, especially in the cancer population. Therefore, the NCCN guidelines do not recommend relying on GCS alone for VTE prophylaxis.1
Role of the Pharmacist
Pharmacists play a major role in selecting, preparing, and dispensing appropriate chemotherapy. Additionally, pharmacists assist in the management of chemotherapy-induced adverse effects such as nausea, vomiting, and pain. It is well established that pharmacist-managed anticoagulation clinics are an effective means of ensuring that cancer patients on warfarin therapy are monitored appropriately.15 Pharmacists are in a position to help risk-stratify cancer patients taking thalidomide and lenalidomide to determine whether VTE prophylaxis is warranted. Pharmacists also can educate cancer patients about the signs and symptoms of VTE and the importance of adhering to VTE prophylaxis regimens. The arrival of new anticoagulants on the market is likely to expand the pharmacist’s role to assisting in the selection of the appropriate anticoagulant for individual patients.
Conclusion
VTE risk is high in ambulatory cancer patients, especially multiple myeloma patients receiving angiogenesis inhibitors. Consensus guidelines currently do not overwhelmingly recommend the widespread application of VTE prophylaxis because of the potential bleeding risk with anticoagulant therapy.1,13 Clinicians must use existing risk-assessment models to identify which patients might benefit most from VTE prophylaxis. Pharmacists can assist with risk stratification and anticoagulant management. Enoxaparin and warfarin remain the primary options for VTE prophylaxis, but a role for the newer oral anticoagulants may develop once supportive evidence becomes available.
REFERENCES
1. Streiff MB, Bockenstedt PL, Cataland SR, et al. Venous thromboembolic disease. Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2011;9:714-777.
2. Weitz JI. Potential of new anticoagulants in patients with cancer. Thromb Res. 2010;125(suppl 2):S30-S35.
3. CancerThrombosis.org. Pathophysiology and natural history of
venous thromboembolism. www.cancerthrombosis.org/moduleArticle?id=2.
Accessed December 28, 2012.
4. Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414-423.
5. Zonder JA. Thrombotic complications of myeloma therapy. Hematology. 2006;2006:348-355.
6. Corso A, Lorenzi A, Terulla V, et al. Modification of
thrombomodulin plasma levels in refractory myeloma patients during
treatment with thalidomide and dexamethasone. Ann Hematol. 2004;83:588-591.
7. Kaushal V, Kaushal GP, Melkaveri SN, Mehta P. Thalidomide protects
endothelial cells from doxorubicin-induced apoptosis but alters cell
morphology. J Thromb Haemost. 2004;2:327-334.
8. Hoshi A, Matsumoto A, Chung J, et al. Activation of coagulation by a thalidomide-based regimen. Blood Coagul Fibrinolysis. 2011;22:532-540.
9. Thalomid (thalidomide) product information. Summit, NJ: Celgene Corp; February 2012.
10. Revlimid (lenalidomide) product information. Summit, NJ: Celgene Corp; May 2012.
11. Kuderer NM, Khorana AA, Lyman GH, Francis CW. A meta-analysis and
systematic review of the efficacy and safety of anticoagulants as
cancer treatment: impact on survival and bleeding complications. Cancer. 2007;110:1149-1161.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical
patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed:
American College of Chest Physicians Evidence-Based Clinical Practice
Guidelines. Chest. 2012;141(suppl 2):e195S-e226S.
13. Lyman GH, Kuderer NM. Prevention and treatment of venous
thromboembolism among patients with cancer: the American Society of
Clinical Oncology Guidelines. Thromb Res. 2010;125(suppl 2):S120-S127.
14. Palumbo A, Cavo M, Bringhen S, et al. Aspirin, warfarin, or
enoxaparin thromboprophylaxis in patients with multiple myeloma treated
with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol. 2011;29:986-993.
15. Jones KL, Barnett C, Gauthier M, et al. Clinical outcomes of a
pharmacist-managed anticoagulation service for breast cancer patients. J Oncol Pharm Pract. 2012;18:122-127.
To comment on this article, contact rdavidson@uspharmacist.com.






