US Pharm. 2023;48(9):HS2-HS11.
ABSTRACT: Venous thromboembolism (VTE) is a potentially life-threatening formation of a thrombus in either the lower extremities or lungs. The obstetric period is a vulnerable time, balancing risk to the fetus versus risk to the mother and walking the fine line of hemorrhage versus thrombus. Due to the limited data available regarding medication safety during the obstetric period, the recommended prophylactic medications for VTE include unfractionated heparin and low-molecular-weight heparin. Pharmacists play a key role in identifying patients who may be at risk of VTE, counseling patients about the potential risks of medications antepartum and postpartum, as well as verifying that patients are receiving the proper dosages of prophylactic medications.
Venous thromboembolism (VTE) is a complication of pregnancy that many providers find difficult to manage. While VTE in obstetric patients is not common, it does affect approximately 0.5 to 2 patients per 1,000 pregnant women.1 VTE is the fifth leading cause of maternal mortality (10.5%) in the United States, preceded by cardiovascular conditions, infections, cardiomyopathy, and hemorrhage.1,2 Factors including medications that cross the placenta or appear in breast milk, and the overall risk of fetal harm, make many practitioners worry about starting prophylactic VTE treatment in expectant mothers.1 However, appropriate VTE prophylaxis in high-risk mothers may be the key to preventing further complications, including myocardial infarction, paralysis, stroke, and death during or after delivery.3
VTE is the formation of a clot within a blood vessel of the lungs and/or lower extremities.4 Eighty percent of VTE-related complications in pregnancy are linked to venous origin.1,3 VTE is hypothesized to have evolved as a compensatory mechanism due to the risk of hemorrhage during the obstetric period.5 Deep vein thrombosis (DVT) is more common, occurring in 80% compared with pulmonary embolism (PE), which occurs in 20% of VTE cases associated with pregnancy.1 The antepartum period increases the risk of VTE by 5-fold in age comparison studies.1 The most vulnerable period for obstetric patients lies in the postpartum period, with an average 15- to 35-fold risk increase.1 During the postpartum period, risk rapidly decreases after the first 3 to 6 weeks but does not return to baseline until 12 weeks postpartum.1
Since 1987, the CDC has been tracking the causes of pregnancy-related mortality using the Pregnancy Mortality Surveillance System.2 This surveillance program shows an increasing trend of pregnancy-related deaths.2 In 1987, 7.2 per 100,000 live births resulted in mortality, and in 2019 the rate increased to 17.6 per 100,000 live births.2 As the rate of pregnancy mortality increases, it is imperative to look at the leading causes of mortality in this population and become familiar with prophylactic and treatment options to decrease the risk of complications.
Pathophysiology of VTE in Obstetrics
Hemodynamic stability during pregnancy is a delicate balance between hemorrhage and hypercoagulability, as both are leading causes of pregnancy-related mortality.2 This balance is maintained by natural procoagulants such as thrombin, von Willebrand factor, reactive oxygen species, tissue factor, plasminogen-activator inhibitor–1, and the opposing anticoagulant factors including thrombomodulin, tissue-type plasminogen activator, antithrombin, and heparin-like proteoglycans.5 Due to the lack of research in pregnant women, there are little analytical data to support the direct pathway of VTE in obstetrics.6 The risk of hemorrhage versus thromboembolism in obstetric patients relies heavily on patient-independent risk factors.7 Risk is also related to hormone changes in pregnancy from what they are in a prepregnancy state.7 These changes can allow for the hypercoagulability of blood, leading to an increased risk of the development of VTE and subsequent complications.6 Estrogen is the primary hormone affected during pregnancy. Constantly increasing circulating estrogen levels increase the viscosity of blood, coagulation factors VII, VIII, and X, as well as other procoagulants previously discussed.6 When an obstetric patient develops VTE, it is often seen as a proximal thrombus when occurring due to the increase in estrogen levels.3 Generally, a DVT that develops during pregnancy is more likely to be large and found in the lower-left extremity; however, the exact mechanism of this trend is unknown.3 Pelvic DVT happens almost exclusively in pregnancy, occurring in 10% of obstetric patients.3 Hypercoagulability of blood during pregnancy leads to an alteration of blood flow, and this process is potentially the most contributory factor to developing VTE during pregnancy.3,8 The theory developed by Rudolf Virchow explains that VTE can be related to three factors: hypercoagulability of the blood, alteration of the blood flow through the vessels, and vascular or endothelial injury.8 The presence of these risk factors is often connected and leads to an increased risk of developing VTE.8 Approximately 80% of patients with VTE have a direct factor that can be linked back to the Virchow triad (see TABLE 1).8
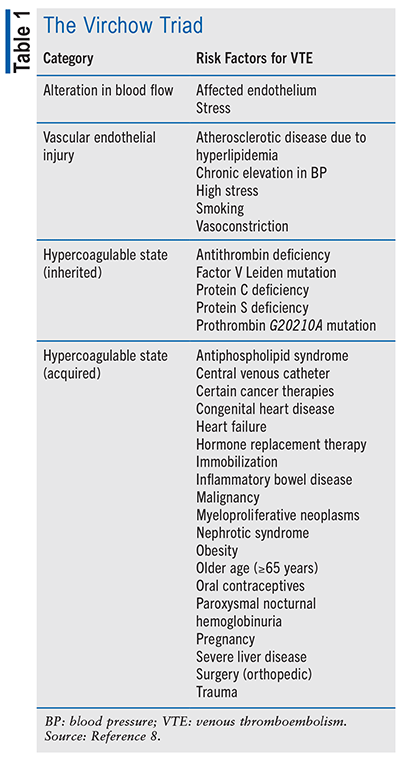
Previous history of VTE is one of the largest risk factors for a woman developing VTE during the obstetric period; up to 25% of events are a result of recurrence. However, with proper anticoagulation, there has been an observed decrease in the recurrence of VTE from 12.2% to 2.4%.3 Other risk factors pertinent to obstetric patients, including thrombophilia, medical conditions increasing the risk of VTE, multiple gestation, hyperemesis, transfusions during pregnancy, postpartum infection, disorders of fluids and/or electrolytes, and cesarean delivery, are found to increase the risk of VTE.5
A study on the incidence, risk, and mortality of VTE during pregnancy that looked at 9,058,162 pregnancies found that age is directly proportional to the risk of VTE development. The study showed the least risk association with women aged 20 to 24 years. As the age increased to 35 years and older, the risk association was much greater than their younger counterparts.5 The same study found that black women are at a higher risk and women of Asian or Hispanic descent were among those least likely to develop VTE during their pregnancy or postpartum period.5
Other proposed mechanisms of coagulation in pregnancy include hypoxia or an inflammatory reaction activating endothelial cells during pregnancy, increasing the response of the coagulation cascade and further decreasing anticoagulation factors typically present.6 Many medications can also impact the factors of the Virchow triad, further tipping the scale of hemostatic balance.6 Medications that can influence the factors in the Virchow triad, thus making patients at higher risk of VTE, include 5-fluorouracil, anthracyclines, everolimus and other mechanistic target of rapamycin inhibitors, selective serotonin reuptake inhibitors, antipsychotics, cyclooxygenase-2 inhibitors, glucocorticoids, heparin, and recombinant human erythropoietin administration.6
Medications
Medication choice for prophylaxis and treatment of VTE is important in the obstetric patient population, as it can be the difference between life and death. Many medications used for anticoagulation without regard to pregnancy have the potential to cross the placenta, transfer to breast milk, or result in teratogenicity.6 Another limitation to choosing treatment during the obstetric period is the lack of clinical studies and human data regarding medication options.6 The ethical restraint of clinical trials during the vulnerable period of pregnancy has limited data to retrospective studies and animal research. The recommended prophylactic medications for both antepartum and postpartum are low-molecular-weight heparin (LMWH) and unfractionated heparin (UFH), according to the American College of Obstetricians and Gynecologists (ACOG), and only LMWH is recommended by the Society of Obstetricians and Gynecologists of Canada.1,9 Dosing of LMWH and UFH is based on low or intermediate dosing strategy; dosages may be adjusted from typical prophylactic dose based on risk level and weight gain associated with pregnancy (see TABLE 2).7
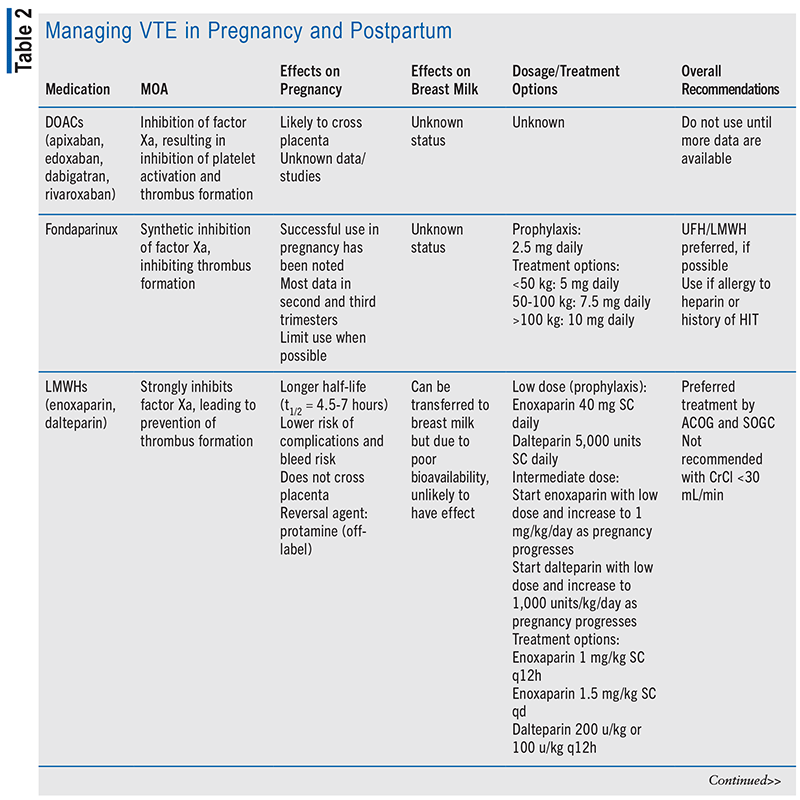
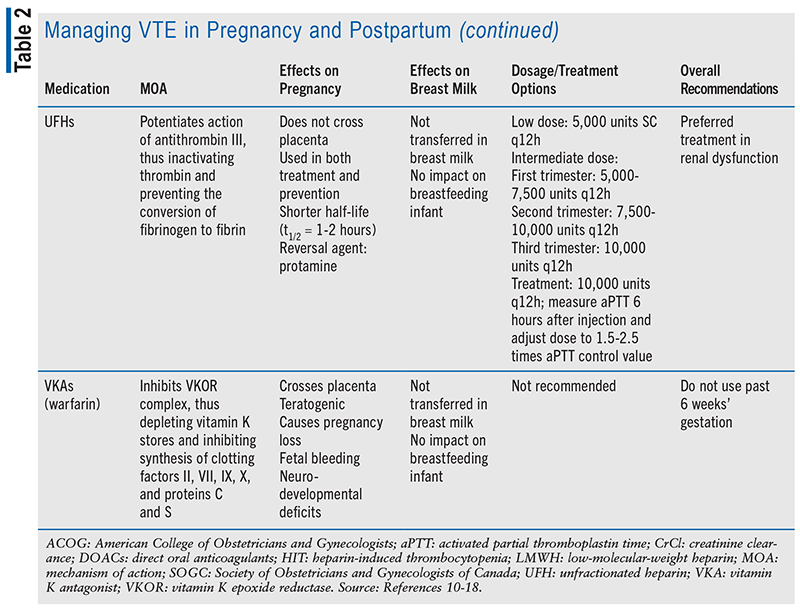
Due to the limited research and the high risk that VTE poses, women at greatest risk of VTE due to mechanical heart valve or history of recurrence of VTE on full anticoagulation should be recommended against pregnancy. Warfarin has been proven to have extensive teratogenic effects and thus is not recommended past 6 weeks’ gestation.1,10 While direct oral anticoagulant (DOAC) medications do not have sufficient data to support their use in pregnancy, many expect the DOAC class of medications, including apixaban, edoxaban, rivaroxaban, and dabigatran, to cross the placenta.11-14 ACOG recommends that any woman taking a DOAC or warfarin of child-bearing age be counseled about the associated potential risks, and regular pregnancy tests are encouraged.15 If a patient develops a DVT 2 to 4 weeks before expected delivery, an inferior vena cava filter should be considered.1
Monitoring
Therapeutic management of anticoagulant medications requires close monitoring of complete blood count (CBC) including platelets, hemoglobin, and hematocrit.16,17 Monitoring for signs and symptoms of bleeding is essential when giving prophylactic or treatment-dose anticoagulation, especially in high-risk populations such as obstetrics.16,17 All obstetric patients regardless of risk status should be monitored for signs and symptoms of thrombosis in both physical and laboratory tests. Testing such as CBC, D-dimer, ultrasound, or CT may be necessary if a thrombus is suspected.6
Prevention in Antepartum
Prophylactic treatment is indicated when the risk of VTE is greater than the potential risks associated with complications and bleeding with either UFH or LMWH.5 The most benefit is seen in patients with a history of thrombosis, as this population is at an increased risk of recurrence.5 Patients with thrombophilia or antiphospholipid syndrome may also benefit from anticoagulation during pregnancy.5 In accordance with ACOG and the American College of Chest Physicians (ACCP), pharmacologic management is recommended in patients with a history of VTE in pregnancy or related to estrogen and in women with recurrent VTE.18 In all pregnancies with a risk of VTE that do not meet the criteria for pharmacologic management, clinical surveillance is recommended, and the patient should be educated on the signs and symptoms of VTE.5 Signs/symptoms to educate the patient on include leg pain/swelling, skin warm to the touch, red discoloration, unexplained shortness of breath, rapid breathing, and chest pain.19 If delivery is expected or scheduled, hold anticoagulation treatment for 24 hours.5 Neuraxial blockade should be held for 10 to 12 hours after the last prophylactic dose of LMWH or 24 hours post therapeutic dose.1
Prevention in Postpartum
The postpartum period holds the most risk of VTE in obstetric patients.1 Patients may opt for a pneumatic device until ambulatory; however, the use is controversial.20 Pneumatic devices in pregnancy do not have sufficient evidence but may modestly decrease the risk of DVT without the risks associated with anticoagulation.20 The CLOTS 3 trial showed that patients recovering from stroke benefited from pneumatic devices while immobile.21 A randomized, controlled trial of 49 patients undergoing cesarean delivery used pneumatic compression devices versus the usual care and found there was no difference in markers of fibrinolysis between the treatment arms.22 Patients surveyed on the drawbacks of using pneumatic devices reported legs becoming hot and sweating as most common, followed by restricted movement and inconvenience.23 If traditional anticoagulation is recommended, medication must not be started before 4 to 6 hours post–vaginal delivery and 6 to 12 hours post-cesarean to minimize bleeding complications associated with delivery.1 The criteria for anticoagulation in the postpartum setting are less stringent due to the increased risk of VTE and no longer having the associated risk of fetal harm.5
Role of the Pharmacist
The role of a pharmacist in the prevention of VTE in obstetric patients lies mostly in the education on the risks associated with anticoagulant medications in women of childbearing age and obstetric patients. Once a patient is started on anticoagulation antepartum or postpartum, the pharmacist will verify that the patient is receiving the correct dose (low, intermediate, or treatment) and duration as appropriate for the period of pregnancy the patient is currently in. Pharmacists also play a role in screening patients who may be at high risk for VTE and recommending clinical surveillance or anticoagulation therapy. Lastly, as pharmacists, consideration must be given to every patient’s current and past medical conditions, medications, and lifestyle to view the patient as a whole and provide patient-centered care regarding VTE prophylaxis.
Conclusion
There are many different VTE risk factors to consider in obstetric patients, such as patient-specific risks, pathophysiologic risk of hypercoagulability, and inherent risks associated with the antepartum or postpartum period. As practitioners, it is essential to identify patients who may benefit from clinical surveillance, pneumatic devices, and/or anticoagulant medications. Identifying high-risk patients and carefully weighing the risks of coagulation and hemorrhage can be the key to decreasing the number of patients who experience VTE and subsequently the number of patients with complications of VTE including paralysis, stroke, myocardial infarction, and death. The overall goal as healthcare providers is providing patient-centered care. VTE in obstetrics is a key example of when considering every factor and taking an interdisciplinary approach is essential for patient outcomes.
REFERENCES
1. Bates SM, Middeldorp S, Rodger M, et al. Guidance for the treatment and prevention of obstetric-associated venous thromboembolism. J Thromb Thrombolysis. 2016;41:92-128.2. CDC. Pregnancy mortality surveillance system. March 23, 2023. www.cdc.gov/reproductivehealth/maternal-mortality/pregnancy-mortality-surveillance-system.htm. Accessed June 22, 2023.
3. James AH. Venous thromboembolism in pregnancy. Arterioscler Thromb Vasc Biol. 2009;29:326-331.4. Bauer KA, Lip GYH. Overview of the causes of venous thrombosis. UpToDate. February 8, 2023. www.uptodate.com/contents/overview-of-the-causes-of-venous-thrombosis. Accessed August 8, 2023.
5. James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006;194(5):1311-1315.6. Struble E, Harrouk W, DeFelice A, et al. Nonclinical aspects of venous thrombosis in pregnancy. Birth Defects Res C Embryo Today. 2015;105(3):190-200.
7. Malhotra A, Weinberger SE. Deep vein thrombosis and pulmonary embolism in pregnancy: prevention. UpToDate. July 19, 2023. www.uptodate.com/contents/deep-vein-thrombosis-and-pulmonary-embolism-in-pregnancy-prevention. Accessed August 8, 2023.8. Kushner A. Virchow triad. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2023 Jan-.
9. Chan W, Rey E, Kent N, et al. Venous thromboembolism and antithrombotic therapy in pregnancy. J Obstet Gynaecol Can. 2014;36(6):527-553.10. Warfarin. In: Lexicomp Online. Waltham, MA: Wolters Kluwer. https://online.lexi.com. Accessed June 2, 2023.
11. Apixaban. In: Lexicomp Online. Waltham, MA: Wolters Kluwer. https://online.lexi.com. Accessed June 2, 2023.12. Edoxaban. In: Lexicomp Online. Waltham, MA: Wolters Kluwer. https://online.lexi.com. Accessed June 2, 2023.
13. Rivaroxaban. In: Lexicomp Online. Waltham, MA: Wolters Kluwer. https://online.lexi.com. Accessed June 2, 2023.14. Dabigatran. In: Lexicomp Online. Waltham, MA: Wolters Kluwer. https://online.lexi.com. Accessed June 2, 2023.
15. Fondaparinux. In: Lexicomp Online. Waltham, MA: Wolters Kluwer. https://online.lexi.com. Accessed June 2, 2023.16. Heparin. In: Lexicomp Online. Waltham, MA: Wolters Kluwer. https://online.lexi.com. Accessed June 2, 2023.
17. Enoxaparin. In: Lexicomp Online. Waltham, MA: Wolters Kluwer. https://online.lexi.com. Accessed June 2, 2023.18. Dalteparin. In: Lexicomp Online. Waltham, MA: Wolters Kluwer. https://online.lexi.com. Accessed June 2, 2023.
19. American Heart Association. Symptoms and diagnosis of venous thromboembolism (VTE). July 22, 2021. www.heart.org/en/health-topics/venous-thromboembolism/symptoms-and-diagnosis-of-venous-thromboembolism-vte. Accessed June 22, 2023.20. Arabi Y, Al-Hameed F, Burns K, et al. Adjunctive intermittent pneumatic compression for venous thromboprophylaxis. N Engl J Med. 2019;380:1305-1315.
21. Dennis M, Sandercock P, Graham C, et al. The clots in legs or stockings after stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess. 2015;19(76):1-90.22. Reddick K, Smrtka M, Grotegut C, et al. The effects of intermittent pneumatic compression during cesarean delivery on fibrinolysis. Am J Perinatol. 2014;31(9):735-740.
23. Reinhard M, Flynn K, Palatnik A. Patient-reported barriers and facilitators for intermittent pneumatic compression device use on the antepartum unit. J Matern Fetal Neonatal Med. 2022;35(26):10388-10394.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





