US Pharm. 2011;36(2):26-43.
Anticoagulation therapy is used to prevent and treat venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE), and prevent stroke and systemic embolism from atrial fibrillation, cardiac valve replacement, or in patients with coronary artery disease.1-3 Warfarin, a vitamin K antagonist (VKA), has been the mainstay of oral anticoagulation therapy for over 50 years. However, warfarin has significant limitations that can impede appropriate use in patients requiring VTE or stroke prevention. Thus, finding an alternative to warfarin has been a focus of recent research. Novel oral agents that inhibit thrombin (factor IIa) or factor Xa, offer more specific treatment effects than that of a VKA, predictable pharmacokinetics to eliminate the need for laboratory monitoring and associated dosage adjustment, and fewer drug and diet interactions.4,5 Recently, dabigatran, the first oral thrombin (factor IIa) inhibitor, became available in the United States, and it offers an alternative oral therapy to warfarin. Other oral anticoagulants, including factor Xa inhibitors, are also being investigated, with anticipated FDA approval in the near future. The pharmacist has a significant role in keeping updated with these new agents and educating, treating, and monitoring patients taking these drugs.
Warfarin
Warfarin, a VKA, has been widely used for the treatment of DVT and PE. Warfarin inhibits vitamin K–dependent clotting factors II, VII, IX, and X and the anticoagulant proteins C and S.6 Initial anticoagulation treatment includes rapid-acting IV unfractionated heparin, subcutaneous (SC) low-molecular-weight heparin (LMWH), or SC fondaparinux given concurrently with warfarin until a therapeutic target international normalized ratio (INR) is reached, which takes approximately 5 to 10 days. Warfarin is also effective in reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.7 The duration of warfarin therapy depends on the indication and the patient’s risk of VTE or stroke, and ranges from 3 months to indefinitely.1,2
Warfarin therapy has a narrow therapeutic range and requires regular laboratory-guided dosage adjustments. In order to maximize the efficacy and minimize the adverse effects of warfarin, the INR should be in the range of 2.0 to 3.0 for most indications. Warfarin is usually initiated at 5 to 10 mg/day, but lower doses should be considered in patients who are elderly, malnourished, have congestive heart disease or liver disease, have had recent major surgery, or are receiving drugs that can increase the effects of warfarin (e.g., amiodarone). An INR should be monitored very closely after the first two to three warfarin doses, and then at weekly intervals, with the monitoring interval extended as the target INR is reached. Once a patient is stable on warfarin therapy, monthly INR monitoring is recommended.8 In general, dose adjustments of warfarin are made by calculating the weekly dose and reducing or increasing it by 5% to 20% depending on the INR value. Warfarin initial dosing and adjustment algorithms are available to guide pharmacists.9,10 Patients should also be monitored for any signs or symptoms of clotting or bleeding.
Anticoagulation management services, which involve patients visiting their clinician’s office regularly for INR testing and warfarin management, are recommended; however, patient self-management can be effective for suitably selected and trained patients. Patient self-management of warfarin and INR concentrations involves the patient monitoring INR using a portable coagulometer. Patients interested in self-management should be trained by an educated health care professional and remain in contact with an identified clinician. Patients should also bring their coagulometer to their clinician’s office regularly for quality control measures to ensure that the device is working properly.8
Warfarin therapy is contraindicated in patients with a hypersensitivity to warfarin, active bleeding, hemorrhagic tendencies or blood dyscrasias, severe liver disease, severe thrombocytopenia, malignant hypertension, an inability to maintain compliance to drug and follow-up monitoring, or pregnancy (Category X). Risk factors of major bleeding should be considered (TABLE 1), and pharmacists should understand how to manage elevated INRs and/or bleeding in patients taking warfarin.6 For the management of elevated INR with or without bleeding, the American College of Chest Physicians antithrombotic therapy guidelines recommend warfarin dosage adjustment or discontinuation, potential vitamin K, fresh frozen plasma, or prothrombin complex administration.8

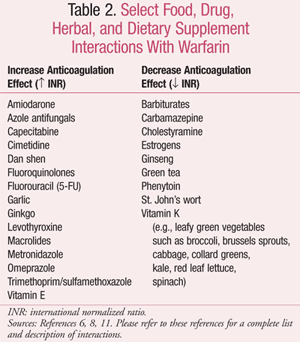
Treatment with warfarin is affected by food and drug interactions, including herbal and dietary supplements (TABLE 2).6,8,11 In a retrospective study, patients using warfarin who received an anti-infective agent showed increased risk of gastrointestinal (GI) bleeding, specifically with concomitant cotrimoxazole and fluconazole.12
Health care providers have a vital role in educating patients on warfarin (see TABLE 3 for a list of patient education tips). Patients should be educated on the importance of taking warfarin correctly, potential adverse effects, and the need to alert a health care provider of any changes in medications or health, in case warfarin dosage adjustment is needed. Follow-up with a health care provider is key in avoiding risk of bleeding or inadequate dosing leading to VTE or stroke, and should be stressed with patients starting and maintained on warfarin therapy.

Dabigatran
Dabigatran etexilate (Pradaxa), the first oral direct thrombin inhibitor, was approved by the FDA on October 19, 2010, for the reduction of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Previous research with direct thrombin inhibitors had been unsuccessful, as made evident by the FDA rejection of ximelagatran (Exanta), which was shown to cause liver injury.4 The same was not seen with dabigatran. Unlike warfarin, dabigatran directly inhibits both free and clot-bound thrombin, works faster, and provides stable anticoagulation at a fixed dose without the need for laboratory monitoring and associated dosage adjustments. Dabigatran etexilate is a prodrug that is rapidly converted to dabigatran, with peak concentrations in 1 to 3 hours, and has a half-life of 12 to 17 hours. It does not undergo metabolism through the CYP450 system, and is eliminated primarily in the urine.13
The phase III Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial evaluated the effects of dabigatran 110 mg or 150 mg twice daily compared to dose-adjusted warfarin (target INR of 2-3) in preventing stroke and systemic embolism in over 18,000 patients with nonvalvular atrial fibrillation and at least one other additional risk factor for stroke or systemic embolism.14 This open-label, multicenter study lasted approximately 2 years and demonstrated that dabigatran 150 mg twice daily was superior to warfarin in lowering stroke and systemic embolism, with similar rates of major bleeding. Dabigatran 110 mg twice daily was noninferior at lowering the risk of stroke; however, it reduced the risk of major bleeding compared to warfarin. Due to concerns that arose with ximelagatran previously, transaminase levels were monitored closely in this trial, and no reports of hepatotoxicity occurred. Dyspepsia was higher in the dabigatran treatment arms compared to warfarin, leading to more patients discontinuing treatment.14
The overall standard of anticoagulation with warfarin therapy in the RE-LY study was a mean time in therapeutic range (TTR) of 64%,14 which was similar to those in other prospective, randomized trials and a recent meta-analysis. As expected with a large study taking place at many locations across the country, there were variations in INR control, which questioned the relevance of the study results to those locations with a better mean INR control. A follow-up, prespecified assessment of the outcomes of the RE-LY trial in comparison to the quality of INR control was recently studied and published.15 Dabigatran’s effects at reducing stroke at 150 mg twice daily, reducing bleeding at 110 mg twice daily, and decreasing intracranial bleeding with both doses in comparison to warfarin were consistent regardless of the centers’ quality of INR control. Dabigatran showed greater benefit for all vascular events, nonhemorrhagic events, and death at study locations with poor INR control than at those with good INR control.15 The RELY-ABLE study is an extension of the RE-LY trial that will evaluate the long-term safety of dabigatran, including liver function, as well as the effect of a knowledge translation intervention on patient outcomes.16
Three large phase III studies have been completed examining dabigatran for the prevention of VTE and mortality after orthopedic surgeries: the RE-NOVATE,17 RE-MODEL,18 and RE-MOBILIZE19 trials. In the RE-NOVATE study, dabigatran 220 mg daily or 150 mg daily was compared to enoxaparin 40 mg SC daily, with the initial dose given preoperatively in patients undergoing total hip replacement surgery. Dabigatran at both doses was noninferior to enoxaparin in lowering VTE and mortality, with similar rates of major bleeding in each group.17 The RE-MODEL study demonstrated noninferiority of dabigatran compared to enoxaparin 40 mg SC daily in reducing VTE and mortality in patients undergoing total knee replacement surgery.18 However, in the RE-MOBILIZE trial, dabigatran was inferior to enoxaparin 30 mg twice daily, administered preoperatively, in lowering VTE and mortality in patients having knee replacement surgery. Differences in dabigatran’s effects between the RE-MODEL and RE-MOBILIZE trials may have been due to variations in study design. In the RE-MOBILIZE study, a higher dose of enoxaparin was used and dabigatran was started later (6-12 hours after surgery as opposed to 1-4 hours in the RE-MODEL study).19 A meta-analysis of the results of these trials confirmed that oral dabigatran is noninferior to SC enoxaparin 40 mg daily, with similar safety.20
In the RE-COVER trial, dabigatran was found to be as effective as warfarin for the treatment of acute VTE, with a similar safety profile.21 Additional studies related to RE-COVER are ongoing. Dabigatran is not currently FDA approved for the prevention of VTE after orthopedic surgeries or for acute VTE; however, it has been approved in Europe and Canada since 2008 for the prevention of VTE after orthopedic surgeries in adult patients.
The most common adverse reactions reported in the RE-LY trial and leading to dabigatran discontinuation were bleeding and GI events (e.g., dyspepsia and gastritis-like symptoms); 21% for dabigatran 150 mg/day and 16% for warfarin-treated patients, respectively. Bleeding occurred in 17% of patients taking dabigatran 150 mg twice daily, and 3% of patients had a major bleeding event. GI adverse events occurred in about one-third of patients taking dabigatran. It is contraindicated in patients with a history of serious hypersensitivity to dabigatran, as well as in active pathologic bleeding. Similar to warfarin, it is essential that patients promptly report any signs of bleeding to a health care provider. Dabigatran should be discontinued before invasive or surgical procedures, if possible, and then restarted soon afterwards. Avoid concomitant administration of dabigatran with P-glycoprotein (Pgp) inducers, including rifampin, due to lowered exposure of dabigatran. No significant interactions with a few Pgp inhibitors have been reported so far; however, data are still somewhat limited.13
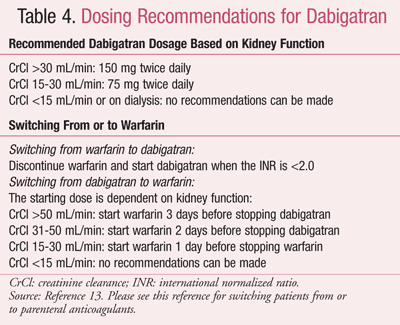
The recommended dosage of dabigatran is 150 mg twice daily for patients with atrial fibrillation.13 (Refer to TABLE 4 for dosage guidelines for patients with impaired kidney function, as well as dosage recommendations when switching from or to warfarin.) Dabigatran can contribute to an elevated INR; therefore, the INR will better reflect warfarin’s effect after dabigatran has been discontinued for at least 2 days. Dabigatran’s product information also reviews how to convert dabigatran from or to parenteral anticoagulants. Dabigatran etexilate is available as 75 mg and 150 mg oral capsules. Interestingly, once the manufacturer’s bottle of dabigatran is opened, it must be used within 30 days. Due to an interaction with moisture, it is recommended that the medication be stored and dispensed in its original container. Individual blister packs of the 150-mg strength are available for outpatient use, with anticipated 75-mg strength availability in the near future, similar to that used in the hospital setting. Patients should be educated not to chew, break, or open the capsule upon administration.13
Dabigatran is significantly more expensive than warfarin. Future cost-effective analyses should account for absence of INR measurements needed with dabigatran therapy. However, it still provides an attractive alternative therapy to warfarin for patients with nonvalvular atrial fibrillation, and perhaps for other indications as well in the future. Dabigatran can be especially helpful for patients needing anticoagulation who do not have access to regular INR monitoring for necessary dosage adjustments, or in patients who have difficulty maintaining the target INR.
Investigational Agents
A number of oral anticoagulants are in phase III studies and may be available in the U.S. in the near future. The first agent is rivaroxaban (Xarelto). Rivaroxaban is a direct inhibitor of coagulation factor Xa and has been available in other countries since 2008. Factor Xa inhibitors do not require cofactors and offer direct inhibition at a critical step of amplification in the coagulation cascade. It is not known if thrombin inhibitors, such as dabigatran, or factor Xa inhibitors will be preferred. Both anticoagulant classes ultimately reduce fibrin formation. Rivaroxaban is similar to dabigatran in that it is rapidly absorbed, and routine coagulation monitoring is not required. Despite their short half-lives, a concern with both classes is the lack of an antidote in the event of bleeding.4
Rivaroxaban 10 mg/day was compared to SC LMWH enoxaparin 40 mg/day or 30 mg twice daily in four clinical studies known as the RECORD (REgulation of Coagulation in ORthopedic surgery to prevent Deep-vein thrombosis and pulmonary embolism) studies, for the prevention of VTE in over 12,000 adult patients having either hip (RECORD1 and RECORD2 studies) or knee replacement surgery (RECORD3 and RECORD4 studies). Rivaroxaban was more effective than enoxaparin and similar in regard to safety measures.22-25
Recently, the results of both the EINSTEIN-Extension and EINSTEIN-DVT studies were published. Rivaroxaban offered a simple and effective therapy compared to enoxaparin followed by a VKA. This trial showed the benefit of a new oral anticoagulant, eliminating the need for initial parenteral therapy followed by oral therapy for the acute management of DVT.26 An EINSTEIN-PE trial evaluating rivaroxaban is still ongoing.27
The ROCKET AF study recently concluded and is awaiting publication, although preliminary information presented at the American Heart Association scientific sessions 2010 demonstrated rivaroxaban as effective as warfarin in reducing stroke and non–central nervous system (non-CNS) systemic embolism in patients with nonvalvular atrial fibrillation.28,29 Bleeding was similar in both study groups; however, serious bleeding rates were lower in the rivaroxaban study group. Additionally, the MAGELLAN trial, using rivaroxaban for the prevention of VTE in hospitalized medically ill patients, has finished and is awaiting study publication.30 Lastly, rivaroxaban added to standard care is undergoing evaluation for the secondary prevention of major cardiovascular events in patients with acute coronary syndrome in the ATLAS ACS-TIMI 51 study.31
Apixaban (BMS-562247-01) is another direct factor Xa inhibitor that showed benefits in patients undergoing knee replacement surgery in reducing thromboembolism compared to enoxaparin without increased bleeding in two phase III trials, ADVANCE-132 and ADVANCE-2,33 and more recently in patients having hip replacement surgery in the ADVANCE-3 study.34 Apixaban is also being investigated in atrial fibrillation (ARISTOTLE35 and AVERROES36) and for VTE treatment (AMPLIFY studies).5 A few other oral direct factor Xa inhibitors being evaluated are edoxaban (DU-176b) and betrixaban (MK-4448).4 Oral direct factor Xa inhibitors are reportedly well tolerated and possibly effective replacements for warfarin. It is a growing area of research for anticoagulation therapy, with other oral therapies in earlier development.
Conclusion
It is an exciting time for pharmacists caring for patients requiring oral anticoagulation therapy. Warfarin remains an important therapy, but has several limitations. Pharmacists can continue to provide patient education, ensure appropriate use, and monitor for efficacy and safety of warfarin. However, with the recent plethora of literature on alternative agents to warfarin and the approval of dabigatran, it will be critical for pharmacists to keep up with these novel agents and their role as they are established in clinical practice. To keep abreast of the latest trial information in anticoagulation therapy, you can visit www.clinicaltrials.gov. Other helpful online resources for pharmacists and patients are listed in Table 5.

REFERENCES
1. Kearon C, Kahn SR, Agnelli G, et al; American College of Chest Physicians. Antithrombotic therapy for venous thromboembolic disease: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest. 2008;133(suppl 6):454S-545S.
2. Singer DE, Albers GW, Dalen JE, et al. Antithrombotic therapy in atrial fibrillation: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest. 2008;133(suppl 6):546S-592S.
3. Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:700-752.
4. Weitz JI, Hirsh J, Samama MM. New antithrombotic drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest. 2008;133(suppl 6):234S-256S.
5. Garcia D, Libby E, Crowther MA. The new oral anticoagulants. Blood. 2010;115:15-20.
6. Coumadin (warfarin) package insert. Princeton, NJ: Bristol-Myers Squibb; January 2010.
7. Andersen LV, Vestergaard P, Deichgraeber P, et al. Warfarin for the prevention of systemic embolism in patients with non-valvular atrial fibrillation: a meta-analysis. Heart. 2008;94:1607-1613.
8. Hirsh J, Guyatt G, Albers GW, et al; American College of Chest Physicians. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th ed). Chest. 2008;133(suppl 6):160S-198S.
9. Ebell MH. Evidence-based initiation of warfarin (Coumadin). Am Fam Physician. 2005;71:763-765.
10. Ebell MH. Evidence-based adjustment of warfarin (Coumadin) doses. Am Fam Physician.
11. Gardiner P, Phillips R, Shaughnessy AF. Herbal and dietary supplement—drug interactions in patients with chronic illness. Am Fam Physician. 2008;77:73-78.
12. Schelleman H, Bilker WB, Brensinger CM, et al. Warfarin with fluoroquin-olones, sulfonamides, or azole antifungals: interactions and the risk of hospitalization for gastrointestinal bleeding. Clin Pharmacol Ther. 2008;84:581-588.
13. Pradaxa (dabigatran) package insert. Ridgefield, CT: Boehringer-Ingelheim; October 2010.
14. Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151.
15. Wallentin L, Yusuf S, Ezekowitz M, et al; RE-LY Steering Committee and Investigators. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalized ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376:975-983.
16. RELY-ABLE long term multi-center extension of dabigatran treatment in patients with atrial fibrillation who completed RE-LY trial. www.clinicaltrials.gov/ct2/ 2005;71:1979-1982.
17. Eriksson BI, Dahl OE, Rosencher N, et al; RE-NOVATE Study Group. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949-956.
18. Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007;5:2178-2185.
19. Ginsberg JS, Davidson BL, Comp PC, et al. Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24:1-9.
20. Wolowacz SE, Roskell NS, Plumb JM, et al. Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following hip or knee arthroplasty. A meta-analysis. Thromb Haemost. 2009;101:77-85.
21. Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342-2352.
22. Eriksson BI, Borris LC, Friedman RJ, et al; RECORD1 Study Group. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765-2675.
23. Kakkar AK, Brenner B, Dahl OE, et al; RECORD2 Investigators. Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet. 2008;372:31-39.
24. Lassen MR, Ageno W, Borris LC, et al; RECORD3 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776-2786.
25. Turpie AG, Lassen MR, Davidson BL, et al; RECORD4 Investigators. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomised trial. Lancet.
26. EINSTEIN Investigators. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med.
27. Oral direct factor Xa inhibitor rivaroxaban in patients with acute symptomatic pulmonary embolism (PE) with or without symptomatic deep-vein thrombosis: Einstein-PE evaluation. http://clinicaltrials.gov/ct2/ 2009;373:1673-1680. 2010;363:2499-2510.
28. ROCKET AF Study Investigators. Rivaroxaban-once daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation: rationale and design of the ROCKET AF study. Am Heart J. 2010;159:340-347.
29. Blast off! ROCKET AF hits Chicago. Cardiobrief. November 15, 2010. http://cardiobrief.org/ 2010/11/15/ blast-off-rocket-af-hits-
30. MAGELLAN—Multicenter, randomized, parallel group efficacy superiority study in hospitalized medically ill patients comparing rivaroxaban with enoxaparin. http://clinicaltrials.gov/ct2/
31. A randomized, double-blind, placebo-controlled, event-driven multicenter study to evaluate the efficacy and safety of rivaroxaban in subjects with a recent acute coronary syndrome. http://clinicaltrials.gov/ct2/
32. Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594-604.
33. Lassen MR, Raskob GE, Gallus A, et al; ADVANCE-2 investigators. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375:807-815.
34. Lassen MR, Gallus A, Raskob GE, et al; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487-2498.
35. Lopes RD, Alexander JH, Al-Khatib SM, et al; ARISTOTLE Investigators. Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J. 2010;159:331-339.
36. Eikelboom JW, O’Donnell M, Yusuf S, et al. Rationale and design of AVERROES: apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. Am Heart J. 2010;159:348-353.
To comment on this article, contact rdavidson@uspharmacist.com.





