US Pharm. 2020;45(1):48-CV3.
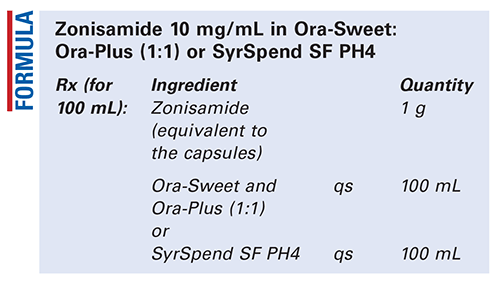
Method of Preparation: Calculate the quantity of each ingredient for the amount to be prepared. Accurately weigh or measure each ingredient. Empty the capsule contents into a mortar and pulverize to a fine powder. Add a small amount of the selected vehicle and mix to form a smooth paste. Geometrically, add the selected vehicle in increments to about 90 mL and transfer to a calibrated container. Add sufficient vehicle to final volume and mix well. Package and label.
Use: Zonisamide is used as an anticonvulsant and as adjunctive therapy in primary generalized tonic-clonic seizures.
Packaging: Package in tight, light-resistant containers.
Labeling: Keep out of reach of children. Keep at room temperature or refrigerated. Shake well before taking. Discard after ____ [time period].
Stability: A beyond-use date of up to 90 days may be used when the preparation is stored either at room temperature or at refrigerated temperature.1,2
Quality Control: Quality-control assessment can include weight/volume, pH (3.9-4.9), specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, or physical stability (discoloration, foreign materials, gas formation, and mold growth).3,4
Discussion: Zonisamide Compounded Oral Suspension, USP, is an official preparation. It is the one that must be prepared if ordered as such, unless there is a valid reason not to do so and the formula presented above is used.
Zonisamide, an antiseizure drug chemically classified as a sulfonamide, is unrelated to other antiseizure agents. Zonisamide (Zonegran, C8H8N2O3S, MW 212.23) occurs as a white to off-white powder that melts at about 163°C. It is tasteless and odorless, has a pKa of 10.2, and is moderately soluble in water (0.80 mg/mL) and 0.1 N HCl (0.50 mg/mL); it is also soluble in methanol, ethanol, ethyl acetate, and acetic acid.5
Zonegran is supplied for oral administration as capsules containing 25 mg or 100 mg zonisamide. Each 25-mg capsule contains the labeled amount of zonisamide plus the following inactive ingredients: microcrystalline cellulose, hydrogenated vegetable oil, sodium lauryl sulfate, gelatin, and titanium dioxide. Each 100-mg capsule contains the labeled amount of zonisamide plus the following inactive ingredients: microcrystalline cellulose, hydrogenated vegetable oil, sodium lauryl sulfate, gelatin, titanium dioxide, FD&C Red No. 40, and FD&C Yellow No. 6.5
Ora-Sweet syrup vehicle is a flavoring vehicle for oral extemporaneous preparations. It is flavored with a citrus-berry flavor blend and contains glycerin and sorbitol to prevent cap lock, a problem associated with many syrups. It is buffered to a pH of approximately 4.2 and has an osmolality of about 3,240 mOsm/kg. Ora-Sweet syrup vehicle contains purified water, sucrose, glycerin, sorbitol (5%), flavoring, sodium phosphate, and citric acid as buffering agents and potassium sorbate and methylparaben as
preservatives.6
Ora-Plus is an oral suspending vehicle that accepts dilution of up to 50% or more with water, flavoring agents, or syrups while still retaining its suspending properties. It has a pH of approximately 4.2 and an osmolality of about 230 mOsm/kg. Ora-Plus is a thixotropic vehicle with a viscosity of approximately 1,000 cps at 25°C. It contains purified water, microcrystalline cellulose, sodium carboxymethylcellulose, xanthan gum, carrageenan, sodium phosphate, and citric acid as buffering agents; simethicone as an antifoaming agent; and potassium sorbate and methylparaben as preservatives.7
SyrSpend SF PH4 is available in liquid form or as a dry powder for reconstitution. The liquid is preserved with less than 0.1% sodium benzoate and is buffered to a pH of 4.2; it is available either unflavored or with cherry flavoring. The powder for reconstitution, which is unflavored, is preservative free and is buffered to a pH of 4.2; it is available as a preweighed powder for making 100 mL (containing 6.5 g powder) or 200 mL (containing 13.0 g powder).8
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; December 2019.
2. Ferreira AO, Polonini HC, Silva SL, et al. Feasibility of amlodipine besylate, chloroquine phosphate, dapsone, phenytoin, pyridoxine hydrochloride, sulfadiazine, sulfasalazine, tetracycline hydrochloride, trimethoprim and zonisamide in SyrSpend(®) SF PH4 oral suspensions. J Pharm Biomed Anal. 2016;118:105-112.
3. Allen LV Jr. Standard operating procedure for performing physical quality assessment of oral and topical liquids. IJPC. 1999;3:146-147.
4. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 1: physical and chemical testing. IJPC. 2019;23:211-216.
5. RxList. Zonegran. www.rxlist.com/zonegran-drug.htm. Accessed December 4, 2019.
6. Ora-Sweet product information. Allegan, MI: Perrigo; 2018.
7. Ora-Plus product information. Allegan, MI: Perrigo; 2018.
8. SyrSpend SF PH4. https://fagron.com/en/product-innovations/syrspend%C2%AE-sf. Accessed February 20, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.





