US Pharm. 2014;39(3):35-38.
ABSTRACT: Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used to treat pain associated with a variety of medical conditions. Nonselective NSAIDs reversibly inhibit the enzyme cyclooxygenase (COX) in both of its isoforms, COX-1 and COX-2. An increased risk of cardiovascular events has been associated with the use of NSAIDs, especially of COX-2 selective NSAIDs. Current evidence suggests that naproxen, a nonselective NSAID, is associated with the lowest risk of cardiovascular events. Therefore, naproxen is the NSAID of choice in patients with high cardiovascular risk.
Cardiovascular disease is defined as any disease that involves the heart, blood vessels, or both, many of which are related to the process of atherosclerosis. Approximately 83 million American adults are affected by one or more types of cardiovascular disease, and of these patients, 40 million are 60 years of age or older.1 Many patients of varying ages utilize OTC and prescription anti-inflammatory drugs to manage pain. Approximately 30 million Americans use nonsteroidal anti-inflammatory drugs (NSAIDs) daily for reasons ranging from cardioprotection and everyday aches and pains to more serious complications such as rheumatoid arthritis, acute gout, and other comorbid conditions.2
Patients at an increased risk for cardiovascular events with concurrent usage of NSAIDs include patients with recent bypass surgery, unstable angina, myocardial infarction (MI), ischemic cerebrovascular events, or any other active athlerosclerotic process. Patients who have cardiovascular disease and are taking NSAIDs, especially cyclooxygenase-2 (COX-2) selective agents, are at a much higher risk of having an MI than patients not taking these drugs. Therefore, understanding the potential danger of the use of NSAIDs in patients who have cardiovascular risk factors is essential.1,3
NSAIDs and Adverse Effects
As with any medication, NSAIDs are not without adverse effects. The most commonly experienced side effects are nausea, vomiting, diarrhea, constipation, decreased appetite, rash, dizziness, headache, and drowsiness. The more severe side effects seen with NSAID use are fluid retention, renal failure (associated with more chronic use), liver failure, gastric ulcers, and increased or prolonged bleeding following an injury or surgical procedure.1,3
Many of the adverse effects associated with NSAID use can be explained by their effect on phospholipid metabolism. Membrane phospholipids are broken down by phospholipase A2 to arachidonic acid, the substrate for the COX enzymes. COX-1 is constitutively expressed in the stomach, kidneys, and intestinal endothelium, where it leads to vasoconstriction and platelet aggregation. COX-2 is upregulated during times of inflammation, where it causes vasodilation and inflammation via the migration of macrophages, leukocytes, and fibroblasts. It is this balance between the two enzymes that is ultimately disrupted upon administration of NSAIDs (FIGURE 1).1,4
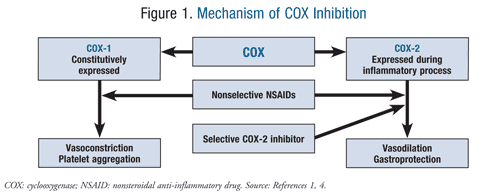
The decision about which NSAID to use will vary among patients, and COX selectivity is one of the determining factors. NSAIDs target both COX-1 and COX-2 enzymes. The rationale for the use of selective COX-2 inhibitors stemmed from the negative gastrointestinal (GI) effects of nonselective NSAIDs and aspirin. Selectivity for the COX-2 enzyme proves to be gastroprotective, which is a major benefit for pain management in patients with GI complications such as peptic ulcer disease (PUD), gastroesophageal reflux disease (GERD), and GI bleeding. However, the COX-2 selective inhibition exhibited by certain NSAIDs can increase the risk of cardiovascular events in patients with preexisting cardiovascular disease. This prothrombotic risk results from thromboxane A2–mediated vasoconstriction and platelet aggregation, which will remain unbalanced and unopposed when prostacyclin activity is suppressed via COX-2 inhibition.1,4
On the other end of the spectrum, irreversible COX-1 inhibition has been shown to be cardioprotective, which is apparent with low-dose aspirin. Nonselective NSAIDs inhibit both COX-2 and COX-1. Because COX selectivity varies among the nonselective NSAIDs, so will the NSAID-associated cardiovascular risk.1,4
Review of Literature
Rofecoxib, a COX-2 inhibitor, has been associated with coronary and cardiovascular death, even in small doses. It was concluded in the Vioxx Gastrointestinal Outcomes Research (VIGOR) trial that there was a great concern for increased cardiovascular risk with rofecoxib.5 It was the Adenomatous Polyp Prevention on Vioxx (APPROVe) trial that ultimately led to the discontinuation of rofecoxib from the U.S. market because it demonstrated the increased risk of acute MI at both low and high doses.6 Current literature estimates a fivefold increase in thromboembolic events in patients taking rofecoxib 50 mg/day compared to naproxen, and a twofold increase in patients taking 25 mg/day compared to placebo.7-9 Shortly after the FDA’s removal of rofecoxib from the market, valdecoxib soon followed suit due to increased risk of MI and stroke.10
In 2005, the FDA concluded that the health benefits of celecoxib outweigh the potential risk in certain informed patient populations.11 As such, celecoxib was left on the market. Pfizer was advised by the FDA to revise the celecoxib label to add a boxed warning and a Medication Guide. The boxed warning states that celecoxib may cause increased risk of serious cardiovascular thrombotic events, MI, and stroke. Celecoxib increases risk at doses higher than 200 mg/day. The risk, if any, that comes with lower doses of celecoxib is not yet clear. The Medication Guide is to be given to patients at the time the drug is dispensed in order to inform them that celecoxib is associated with an increased risk of cardiovascular events and should not be used in patients with high cardiovascular risk.8,12,13
Of note, several other NSAIDs increase cardiovascular risk including the semiselective NSAIDs diclofenac and meloxicam, and the nonselective NSAIDs ibuprofen and naproxen (FIGURE 2).3 Several meta-analyses and systematic reviews indicate that diclofenac has demonstrated the highest cardiovascular risk of any of the nonselective NSAIDs.7,8,12,13 Additionally, in a large retrospective cohort study conducted by Ray et al, when compared to ≥1,000 mg/day of naproxen, diclofenac was associated with an increased risk for MI, stroke, and all-cause mortality in doses <150 mg/day.8 This comes as no surprise when considering the degree of COX-2 selectivity that diclofenac possesses.3 Due to the limited number of trials assessing meloxicam, cardiovascular risk associated with its use remains unknown.14 An evidence-based review of the cardiovascular risk of NSAIDs by Farkouh and Greenberg suggests that ibuprofen is associated with a cardiovascular risk comparable to that of celecoxib.7 In several head-to-head clinical trials, naproxen was not associated with an increased cardiovascular risk in patients with preexisting cardiovascular disease when compared to other nonselective NSAIDs and COX-2 inhibitors. Naproxen has been determined to be the preferred nonselective NSAID for patients with high cardiovascular risk.8,15
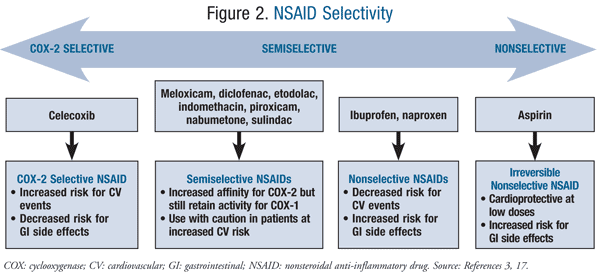
In addition to recognizing the cardiovascular risk profile and dose relationship of each NSAID, it is also important to review the evidence with respect to the timing and duration of therapy. A nationwide cohort study of patients with a first-time MI demonstrated that short-term and long-term use of NSAIDs and COX-2 inhibitors increased the risk of death or recurrent MI.12 A follow-up analysis of this nationwide cohort concluded that there is an increased risk of death and coronary death or nonfatal recurrent MI with the use of any NSAID that persists for at least 5 years after the first MI.13 Overall, the evidence suggests advising caution against both short-term and long-term use of NSAIDs and selective COX-2 inhibitors in patients with high risk of cardiovascular disease.
The Prospective Randomized Evaluation of Celecoxib Integrated Safety vs. Ibuprofen or Naproxen (PRECISION) trial is currently under way.16 It is a randomized, double-blind, parallel-group study comparing the cardiovascular risk associated with celecoxib to that of the two most commonly prescribed nonselective NSAIDs (naproxen and ibuprofen) in patients with either osteoarthritis or rheumatoid arthritis. The primary outcome of the study is the first occurrence of cardiovascular death, nonfatal MI, or nonfatal stroke. Cardiovascular, GI, and renal side effects as well as symptomatic relief will be assessed in the study. The trial is set to be completed in September 2015 and may provide additional evidence for the use of NSAIDs in patients with cardiovascular risk.16
American Heart Association Stance
In 2007, the American Heart Association (AHA) addressed the concern that selective COX-2 inhibition may potentiate a cardiovascular event in patients who are at an increased risk. The AHA states that physicians and patients must weigh the risks and benefits of each agent before choosing a treatment plan for pain relief. Patients should be treated only for the shortest amount of time and with the lowest dosage of drug necessary to gain symptom relief.13,17
A stepwise approach is suggested, beginning with the agents that have the lowest associated cardiovascular risk and moving to the agents with higher risk if treatment failure occurs. Non-NSAID products, such as acetaminophen or nonacetylated salicylates, are the preferred agents in patients with high cardiovascular risk. If these treatments are not tolerated or fail to control pain, the use of NSAIDs may be warranted. Patients beginning NSAID therapy should start with a nonselective NSAID, such as ibuprofen or naproxen. However, the literature suggests naproxen as the nonselective NSAID of choice for these patients. If pain control is not established with nonselective NSAIDs, the next trial should be with an agent that is semiselective for COX-2, such as meloxicam or diclofenac.17
Finally, if all the above treatments have failed, the patient may consider treatment with the selective COX-2 inhibitor celecoxib. Celecoxib use in patients at risk for thrombotic events should be reserved for patients who have no other appropriate alternatives.17
Concurrent use of nonselective NSAIDs with aspirin may decrease the cardioprotective profile normally seen with aspirin use. This is due to competitive inhibition of the receptor-binding site of the COX-1 enzyme. The Therapeutic Arthritis Research and Gastrointestinal Event Trial (TARGET) concluded that ibuprofen was shown to negate the cardioprotective effects of aspirin.7 Although no cardiovascular interaction has been seen with concomitant use of celecoxib and aspirin, administration of aspirin with a selective COX-2 inhibitor may negate the gastroprotective effects of selective COX-2 inhibition. Despite these concerns, patients using low-dose aspirin for cardioprotection may take NSAIDs. Aspirin’s cardioprotective profile may help to decrease the cardiovascular risk associated with NSAID or COX-2 inhibitor use. Furthermore, the addition of a proton pump inhibitor (PPI) may be warranted in certain patients with high GI risk to prevent ulcers and other GI side effects associated with concurrent aspirin and NSAID or COX-2 inhibitor use.14,17,18
During treatment with NSAIDs, patients should be closely monitored for increases in blood pressure, signs of edema, decreasing renal function, and GI bleeding. If any of these adverse effects occur, a decrease in dose or a change in drug may be warranted. With each patient case the risks and benefits of treatment should be considered before beginning any one agent. It should be noted that all NSAIDs, including COX-2 inhibitors, can raise blood pressure in some patients. When initiating any NSAID in a patient with high cardiovascular risk, it is important to monitor blood pressure. Concurrent use of NSAIDs with antihypertensive medications has been associated with a decrease in the blood pressure lowering effects of the antihypertensive therapy.1,7,17
Pharmacist’s Role
The significance of pharmacist-patient communication is crucial in informing patients about the risks associated with use of NSAIDs. It is important for pharmacists to monitor patient profiles for NSAID usage as well as to counsel patients with existing cardiovascular disease about the use of OTC NSAIDs. Due to the wide availability of OTC NSAID products, patients may assume they are safe for all and be unaware of the increased cardiovascular risk. These situations provide an opportunity for pharmacist intervention to educate patients on the risk and inform them of preferred alternative therapies for pain management, such as acetaminophen, capsaicin, and other topical products.1,17
Notably, healthcare professionals should keep in mind that for patients with arthritis or other comorbid conditions who require NSAID therapy, naproxen appears to be the safest from a cardiovascular perspective. For patients at high risk of NSAID-related GI tract complications, naproxen plus a PPI is less costly, safer, and as effective as low-dose celecoxib. The cardiovascular risk with NSAIDs appears to increase as the dosage and duration of therapy increase. Patients receiving NSAID therapy should be advised to use the lowest effective dose for the shortest duration of time.12,17
REFERENCES
1. CDC. Heart Disease and Stroke Prevention: Addressing the Nation’s Leading Killers. At a Glance 2011.
Atlanta, GA: CDC; 2011.
www.cdc.gov/chronicdisease/resources/publications/aag/pdf/2011/Heart-Disease-and-Stroke-AAG-2011.pdf.
Accessed November 25, 2013.
2. Osteoarthritis Health Center. WebMD. www.webmd.com/osteoarthritis. Accessed November 25, 2013.
3. Chisholm-Burns M, Schwinghammer T, Wells B, et al. Pharmacotherapy Principles and Practice. 3rd ed. Columbus, OH: McGraw-Hill; 2013.
4. Brunton L, Chabner B, Knollman B, et al. Goodman and Gillman’s The Pharmacological Basis of Therapeutics. 12th ed. Columbus, OH: McGraw Hill; 2011.
5. Bombardier C, Laine L, Reicin A, et al; VIGOR Study
Group. Comparison of upper gastrointestinal toxicity of rofecoxib and
naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000;343:1520-1528.
6. Garcia Rodriguez LA, Tacconelli S, Patrignani P. Role
of dose potency in the prediction of risk of myocardial infarction
associated with nonsteroidal anti-inflammatory drugs in the general
population. J Am Coll Cardiol. 2008:52:1628-1636.
7. Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol. 2009;103:1227-1237.
8. Ray WA, Varas-Lorenzo C, Chung CP, et al.
Cardiovascular risks of nonsteroidal anti-inflammatory drugs in patients
after hospitalization for serious coronary heart disease. Circ Cardiovasc Qual Outcomes. 2009;2:155-163.
9. Solomon SD, Wittes J, Finn PV, et al. Cardiovascular
risk of celecoxib in 6 randomized placebo-controlled trials: the cross
trial safety analysis. Circulation. 2008;117:2104-2113.
10. Nussmeier NA, Whelton AA, Brown MT, et al.
Complications of the COX-2 inhibitors parecoxib and valdecoxib after
cardiac surgery. N Engl J Med. 2005;352:1081-1091.
11.
Public Health Advisory. FDA
announces important changes and additional warnings for COX-2 selective
and nonselective nonsteroidal anti-inflammatory drugs (NSAIDs). April 7,
2005.
www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm150314.htm.
Accessed November 25, 2013.
12. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al.
Duration of treatment with nonsteroidal anti-inflammatory drugs and
impact on risk of death and recurrent myocardial infarction in patients
with prior myocardial infarction. Circulation. 2011;123:2226-2235.
13. Schjerning Olsen AM, Fosbol EL, Lindhardsen J, et al.
Long-term cardiovascular risk of NSAID use according to time passed
after first-time myocardial infarction: a nationwide cohort study. Circulation. 2012;126:1955-1963.
14. McGettigan P, Henry D. Cardiovascular risk and
inhibition of cyclooxygenase: a systematic review of the observational
studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006;296:1633-1644.
15. Graham DJ. COX-2 inhibitors, other NSAIDs, and cardiovascular risk the seduction of common sense. JAMA. 2006;296:1653-1666.
16. Prospective Randomized Evaluation of Celecoxib
Integrated Safety vs. Ibuprofen or Naproxen (PRECISION).
ClinicalTrials.gov. November 21, 2013.
http://clinicaltrials.gov/ct/show/NCT00346216?order=4. Accessed November
24, 2013.
17. Antman EM, Bennett JS, Daugherty A, et al. Use of
nonsteroidal anti-inflammatory drugs: an update for clinicians: a
scientific statement from the American Heart Association. Circulation. 2007;115:1634-1642.
18. Kearney PM, Baigent C, Godwin J, et al. Do selective
cyclo-oxygenase-2 inhibitors and traditional nonsteroidal
anti-inflammatory drugs increase the risk of atherothrombosis?
Meta-analysis of randomized trials. BMJ. 2006;332:1302-1308.
To comment on this article, contact rdavidson@uspharmacist.com.





