US Pharm. 2015;40(5):22-26.
ABSTRACT: Enterovirus D68 (EV-D68) is a highly contagious viral illness that causes symptoms of respiratory infection. Although this virus is capable of infecting adults and children, pediatric patients with preexisting respiratory conditions such as asthma are at the greatest risk of developing the most serious adverse effects. Pharmacists can play a significant role in promoting opportunities to prevent and limit the spread of infection. They may also offer clinical recommendations for OTC supportive care for mild illness, referrals to confirm diagnosis, and advice to seek immediate medical intervention for patients exhibiting more severe manifestations of this infection. There is currently no vaccine available to prevent EV-D68 infection.
Among the top media stories in 2014, news of the emergence of highly virulent viruses was clearly some of the most disturbing. One of the most notable viral outbreaks in 2014 was responsible for sending hundreds of children to the hospital, alarming parents and putting area schools on high alert for a more immediate threat—a dangerous and potentially deadly illness that could infect school children across the United States.1 Infection with enterovirus D68 (EV-D68) starts like the common cold and shares similar respiratory symptoms across the course of illness. The clinical presentation of this infection in confirmed outbreaks has ranged from mild symptoms to severe illness, with medical complications often requiring hospitalization and, in some cases, causing death.
Background
EV-D68 was first discovered in California in 1962 when it was isolated from samples obtained from children with pneumonia.1 The higher rate of severe cases in children when compared to adults is likely due to the physiological difference of smaller airways and less previously established immunity. The CDC has confirmed a total of 1,153 people in 49 states and the District of Columbia with respiratory illness caused by EV-D68 from mid-August 2014 to January 15, 2015, with 14 deaths among the cases.1 According to Susan Gerber, MD, team lead of the Respiratory Viruses and Picornavirus Team, Division of Viral Diseases, at the CDC, “Most people who contract EV-D68 will get better, but for those with an underlying comorbid respiratory illness there can be risk of severe respiratory illness. Children who have asthma or a history of wheezing are at increased risk of severe respiratory illness.”2
Enteroviruses come from the Picornaviridae family and Enterovirus genus and are nonenveloped, “positive sense,” single-stranded RNA (ssRNA) viruses.3 EV-D68 is epidemiologically and biologically similar to the human rhinovirus.3 Roughly 100 serotypes of nonpolio enteroviruses have been recognized, but EV-D68 has been one of the less commonly reported. Due to the fact that humans are the only known host, these enteroviruses are described as human and are further classified as echoviruses, polioviruses, and coxsackieviruses group A and B. Although there are now more species recognized, the original division of the human enteroviruses (HEVs) placed the viral characteristics into four species: HEV types A, B, C, and D. HEV-68 has now been renamed EV-D68, reflecting the recent revision in taxonomy that removed the “human” host description from both enterovirus and rhinovirus species and added the type number to the species designation.3 Of the currently recognized species of HEVs, EV-D68 is most similar to the human rhinovirus with regard to its ability to cause respiratory illness.
Although it is not a new illness, EV-D68 has become an emerging pathogen responsible for causing outbreaks internationally. Tokarz et al studied the increased prevalence and/or recognition of this illness and reported that the EV-D68 genome experienced a mutation in the protein arrangement, which resulted in a protein deletion in all the strains that were examined.3 In the 1990s, a further viral mutation and protein deletion occurred, which affected the viral replication, virulence, fitness, and persistence. These changes are thought to have improved the viral translation/replication efficiency and may be correlated with the recent increases in EV-D68 cases internationally.3
The CDC obtained one complete genomic sequence and six nearly complete genomic sequences from viruses representing the three known strains of EV-D68 responsible for infections during the current outbreak. These sequences are genetically related to previous strains. These samples have been submitted to GenBank to make the sequences available for further testing. GenBank is the genetic sequence database of the National Institutes of Health, storing a collection of all publicly available DNA sequences.4
According to Dr. Gerber, “There are many different types of enteroviruses, and different types generally predominate seasonally in summer and fall. The CDC has identified smaller EV-D68 outbreaks in previous years. However, it is thought that increased surveillance and awareness has led to the larger number of recognized diagnoses of EV-D68 infection. It is possible, and most likely, that many cases and outbreaks of EV-D68 and other enterovirus-associated illnesses have occurred but have not undergone specific testing. Additionally, there could be a detection bias in certain outbreaks due to the observed severity of illness because testing is more likely with severe cases. It is expected that a high percentage of cases of EV-D68 would be identified among intensive care patients and those requiring the most intensive supportive care. There have been new reports of fatalities in the current outbreak.”2
“This illness does not require Department of Health or CDC notification; therefore, it is difficult to track with any degree of accuracy,” said Dr. Gerber.2 “Not all labs have the ability to test to confirm this infection, making benchmarking difficult, so better information collection is needed for future comparisons. Enteroviruses can cause a wide variety of illnesses, including hand-foot-and-mouth disease with a rash as a distinguishing feature, aseptic meningitis, and conjunctivitis. Pharmacists should be aware of the potential for EV-D68 to cause respiratory illness, and there is no approved vaccination or antiviral for use. There continues to be interest in increasing more research in this area, so there is a wider base of knowledge to apply in practice.” Please refer to the Sidebar for additional content from the interview with Dr. Gerber.1-3,5,6
Interview With Susan Gerber, MD2
Additional questions answered by Dr. Gerber, team lead of the CDC’s Respiratory Viruses and Picornavirus Team
Question: You mentioned that not all labs can test for this illness easily. Is this based on expense, or is the test not easily accessible by all? On October 14, 2014, the CDC press release stated it had developed and started using a new, faster lab test for detecting EV-D68 in specimens from people in the U.S.5 This was reported to be a real-time lab test that uses a reverse transcription polymerase chain reaction, or rRT-PCR, and is expected to identify all strains of EV-D68 that have been reported and documented in the U.S. this summer and fall. Have any additional tests been developed since then?
Answer: Many hospitals and some doctors’ offices can test sick patients to see if they have enterovirus infection. However, most cannot do specific testing to determine the type of enterovirus, like EV-D68. The CDC and some state health departments can do this sort of testing. There have been no new tests to identify EV-D68 since the CDC developed and starting using the new rRT-PCR test on October 14, 2014. The CDC has made the protocols for this lab test publicly available on its website [www.cdc.gov/non-polio-enterovirus/hcp/EV-D68-hcp.html]6 and is exploring options for providing test kits to state public health labs.
Question: This new test was expected to increase the total number of cases confirmed, from the 1,000 remaining samples collected and stored from September. Did this occur?
Answer: Yes, the CDC was able to test the remaining specimens in the backlog, which resulted in an increase in confirmed cases.
Question: Do we know anything more now compared to October 2014 when the story first broke?
Answer: Pharmacists can check the CDC’s EV-D68 website [www.cdc.gov/non-polio-enterovirus/about/ev-d68.html]1 and stay tuned for summary information about the outbreak on our website soon. We know that basically the entire country was affected by the outbreak (at least one person from 49 out of 50 states tested positive for EV-D68). The CDC, working with state health departments, identified at least three separate strains of EV-D68 that caused infections in the U.S. in 2014. It is common for multiple strains of one enterovirus type to cocirculate in the same year. We also know that the strains of EV-D68 circulating this season are not new. The most prominent strain has been in the U.S. since 2012; it was not a recent introduction. It is also related to strains of EV-D68 detected in previous years in countries in Europe and Asia.
Question: The current EV-D68 strain is genetically related to strains from previous years, but do you agree with the Tokarz group,3 who have reported protein deletions that could have contributed to viral fitness and efficiency?
Answer: We generally do not comment on studies done by others. We attribute the increase in cases this year to increased surveillance and awareness, and more testing.
Question: According to the CDC, 1,121 cases of this virus have been lab-confirmed in 47 states from the middle of August to November 20, 2014. Is this different now?
Answer: The data you have in the body of your story through December 18th are correct and the most current. There are continual updates visible on the EV-D68 website.1
Question: The CDC has collected and tested over 2,500 specimens—40% have been positive for EV-D68, 30% have been rhinovirus or enterovirus other than EV-D68. What was the other 30%?
Answer: The other 30% was negative for EV-D68 and any other enterovirus or rhinovirus.
Question: Is the severity of this outbreak greater than in the past?
Answer: U.S. healthcare professionals are not required to report known or suspected cases of EV-D68 infection to health departments because it is not a nationally notifiable disease in the U.S. Also, the CDC does not have a surveillance system that specifically collects information on EV-D68 infections; no data are currently available regarding the overall burden of morbidity or mortality from EV-D68 in the U.S. In essence, we don’t have data to compare this season with previous years. However, infections were widespread with confirmed EV-D68 infection among severe cases in 49 out of 50 states, as of December 18, 2014.
Respiratory Illness
The current EV-D68 outbreak started in the U.S. in August 2014, with two pediatric hospitals reporting an increased rate of admissions for severe respiratory illness and increased infections confirmed by polymerase chain reaction (PCR) assay of nasopharyngeal specimens.7 Respiratory illness is not the only physical manifestation of illness, and other symptoms are discussed in the following section.
During late summer and fall 2014, enteroviruses and rhinoviruses likely contributed the most to the increases in respiratory illnesses. During 2014, the most common type of enterovirus detected was the EV-D68.1 Many viruses can cause similar respiratory illnesses and can only be identified and differentiated through laboratory testing. However, many people with respiratory illness who go to the doctor are not tested, and often those with mild respiratory illness do not seek medical attention. At the end of the fall season, enterovirus infections began to decline as expected, and at the same time respiratory illnesses caused by other viruses, such as influenza, became more common. The cause of this seasonal change in viral prevalence is likely due to the temperature sensitivity of EV-D68 and results in a greater diagnostic challenge for providers.8 Overall respiratory illnesses may appear to be the same; however, the sources of the viral infection may be entirely different.
The incubation period of EV-D68 is between 1 and 5 days, which is similar to that of other enteroviruses and rhinoviruses, and a person is considered contagious from 1 day prior to symptom onset to 5 days after symptoms appear.3 The virus is found in nasal mucus, sputum, and saliva and is spread most likely via droplets expelled with coughs and sneezes of an infected person. The oral-fecal route is another likely means of transmission via unwashed hands and contaminated objects and surfaces. As previously noted by Dr. Gerber, there are no vaccines or antivirals to prevent infection at this time.2
Neurologic Illness
On September 12, 2014, the CDC was notified by the Colorado Department of Public Health and Environment that a number of children had presented with acute neurologic illness including weakness, cranial nerve dysfunction, diplopia, facial droop, dysphagia, and dysarthria between August and September 2014.9 The age range of affected patients was reported to be 1 to 18 years, with the median age of 8 years. All had symptoms of febrile illness 3 to 16 days before the onset of neurologic symptoms.
On September 26, 2014, the CDC released a health advisory based on its investigation of the 10 pediatric patients hospitalized as a result of this neurologic illness, noting that this investigation explored the potential link between these symptoms and the EV-D68 viral outbreak.10 Four of the 8 children tested positive for EV-D68. From mid-August 2014 to January 15, 2015, the CDC or state public health laboratories confirmed a total of 1,153 people in 49 states and the District of Columbia with respiratory illness caused by EV-D68.1 According to the CDC, almost all of the confirmed cases were among children, many of whom had asthma or a history of wheezing, while millions of others with only mild symptoms of EV-D68 infection did not even seek medical treatment or testing.
Patients aged ≤21 years with acute onset of focal limb weakness that occurred on or after August 1, 2014, as well as an MRI showing a spinal cord lesion restricted to the gray matter, were eligible to be included in this investigation. In most cases, recovery occurred within a few days of supportive care, including intensive care and assisted ventilation.9
High-Risk Groups
Pharmacists should be aware of the specific groups, especially children, who are at high risk for contracting EV-D68, including7:
- Children aged ≤4 years
- Children with a history of respiratory disease (e.g., wheezing, asthma, bronchiolitis), especially those with more severe illness
- Both children and adults with immunocompromising conditions.
Children with asthma are particularly at risk for severe symptoms from EV-D68 infection. Therefore, if a child has asthma, parents must take steps to prepare in case the child becomes ill with EV-D68. The CDC recommends the following to control a child’s asthma during this time1:
- Discuss options to develop and update the child’s asthma action plan with his or her physician
- Educate parents about the importance of adhering to treatment and ensuring the child takes his or her prescribed asthma medications as directed, especially long-term control medications for persistent asthma Children with less severe persistent asthma may require medications such as low-dose inhaled corticosteroids, mast cell stabilizers (cromolyn), and/or an oral leukotriene receptor antagonist such as montelukast (Singulair). More severe illness requires more intense steroidal intervention, and often includes oral therapy. Children with intermittent asthma may be controlled with short-acting bronchodilators used only when needed; however, overall use should be monitored and increased use should be promptly reported to the pediatrician to rule out a more serious respiratory exacerbation11
- Remind parents that the child should receive the annual influenza vaccine, since flu and other respiratory infections can trigger an asthma attack
- Refer parents and families to their healthcare provider immediately if the child develops new or worsening asthma symptoms
- Educate caregivers and/or teachers so that they are aware of the child’s condition and know how to help if the child experiences any symptoms related to asthma.
Role of the Pharmacist
Pharmacists are in a good position to provide public education about EV-D68. All patients should be vaccinated to prevent influenza early in the season, and those at risk should receive the pneumococcal vaccine. This will reduce the chance of acquiring the influenza virus and, although not 100% effective against all possible strains, vaccination will assist in triage of the diagnosis of possible respiratory illness.
Patients should not insist on getting an antibiotic when discussing treatment options with their prescribing provider. Antibiotics are not effective against the EV-D68 or any other viral illnesses, and overuse or misuse of antibiotics contributes to resistance. Each year in the U.S., at least 2 million people become infected with bacteria that are resistant to antibiotics, and at least 23,000 people die each year as a direct result of these infections.12
Proper hand hygiene is important in the prevention of viral transmission. Patients should wash hands frequently with soap and water after using the bathroom, after changing diapers, and before and after touching their eyes, nose, or mouth. Patients should also avoid touching their eyes, nose, and mouth with unwashed hands, sharing utensils or drinking cups with people who are sick, having close contact with others (e.g., hugging and kissing), and attending work or school if sick. Frequently touched surfaces such as doorknobs and toys should be disinfected if someone is sick.
Supportive Care: Supportive care is also an important element of treatment and plays a role in providing comfort to patients, even for mild cases. Antipyretics and pain relievers, such as acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs; e.g., ibuprofen) can be used for adults and children. In order to improve safe and effective use of these products, parents and caregivers should be instructed to use a calibrated oral syringe (or measuring cup) when administering these products to children. Measuring devices are often included with the product, and those administering medication should be reminded to always read and follow the directions provided on the label before giving a dosage, regardless of the brand used. The FDA notified the public in 2011 that an additional concentration of liquid acetaminophen marketed for infants (160 mg/5 mL) is now available OTC. Prior to this release, liquid acetaminophen marketed for infants was only available in 80 mg/0.8 mL or 80 mg/mL concentrated drops.13
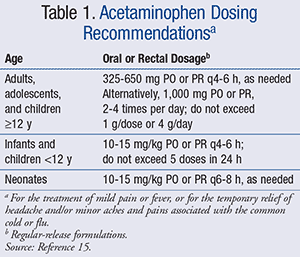
That same year, the FDA announced a joint meeting of the Nonprescription Drugs Advisory Committee and the Pediatric Advisory Committee to discuss the OTC use of acetaminophen in children. Based on recommendations made by this joint committee, the Consumer Healthcare Products Association (CPHA), a national trade association representing the leading manufacturers and distributors of OTC medication, took further action by voluntarily discontinuing the concentrated drops and only supplying the standard liquid strength available to children aged ≥2 years (180 mg/5 mL).14 OTC dosing guidelines recommend confirming appropriate doses for those <2 years of age and limiting the total number of doses to no more than 5 in a 24-hour period.13 However, if providers seek pharmacist recommendation for dosing in other age groups, the following dosing guidelines can be provided (TABLE 1).15 Parents and caregivers should be reminded not to use acetaminophen for children for longer than 5 days and not use it for extremely high fevers (defined as temperature >39.5˚C, or 103.1˚F), fever persisting longer than 3 days, or recurrent fever, unless directed by a prescriber.
Aspirin and combinations containing aspirin or aspirin-like products, including Pepto-Bismol and Kaopectate, should be avoided in pediatric patients due to the increased risk of Reye syndrome. Although a causal relationship has not been entirely defined, this risk is associated with aspirin administration to children with viral illness.15
Nasal saline spray 0.65% (e.g., Ocean Saline Nasal Spray) can be used as often as needed or as directed by a prescriber to loosen congestion. Commercially available dispensing squeeze bottles can be used as a drop for infants and a spray for older children.16 Oral nasal decongestants are not recommended for patients with hypertension, and topical decongestant sprays can cause rebound congestion when used for prolonged periods. Multipurpose products often contain ingredients that are unnecessary and may not be appropriate for children; only single-ingredient products, which are symptom-specific and appropriate, should be used.
The management of severe cases should be conducted only under the direct care of a prescriber, and often requires hospitalization. Any patient who presents with severe symptoms, including wheezing and difficulty breathing, should be referred for emergency care immediately.17
Conclusion
EV-D68, a recognized infectious entity since the 1960s, has recently experienced a surge of identified cases. With improved laboratory testing technology now available to confirm diagnosis, scientists have suggested that this surge is more about “accurate disease confirmation” rather than an increase in overall incidence of EV-D68. While infection control specialists recognize that this infection continues to be a public health concern, the current focus is on standard viral infection precaution and prevention, with no news of novel vaccine or antiviral development targeted specifically for this illness on the horizon.
REFERENCES
1. CDC. Enterovirus D68. Updated March 23, 2015. www.cdc.gov/non-polio-enterovirus/about/ev-d68.html. Accessed April 8, 2015.
2. In-person interview by Tammie Lee Demler with Dr. Susan Gerber, team lead of the Respiratory Viruses and Picornavirus Team at the CDC, on January 6, 2015.
3. Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. J Gen Virol. 2012;93: 1952-1958.
4. National Center for Biotechnology Information (NCBI). GenBank overview. www.ncbi.nlm.nih.gov/genbank. Accessed January 6, 2015.
5. CDC develops a new, faster lab test for Enterovirus D68. October 14, 2014. www.cdc.gov/media/releases/2014/p1014-test-enterovirus-D68.html. Accessed April 8, 2015.
6. CDC. Enterovirus D68 for health care professionals. Updated March 23, 2015. www.cdc.gov/non-polio-enterovirus/hcp/EV-D68-hcp.html. Accessed April 8, 2015.
7. National Collaborating Centre for Infectious Diseases. Disease debrief: EV-D68. What do we know about the current and previous outbreaks of Enterovirus D68 (EV-D68). Updated January 19, 2015. www.nccid.ca/disease-debrief-ev-d68#Q1. Accessed April 8, 2015.
8. Liu Y, Sheng J, Fokine A, et al. Structure and inhibition of EV-D68, a virus that causes respiratory illness in children. Science. 2015;347(6217): 71-74.
9. Colorado Department of Public Health and Environment. Health advisory: severe respiratory illness associated with Enterovirus D68. September 12, 2014. https://www.colorado.gov/pacific/sites/default/files/DC_ComDis-HAN-Severe-Respiratory-Illness-Associated-with-Enterovirus-D68.pdf. Accessed April 8, 2015.
10. CDC health advisory. Acute neurologic illness with focal limb weakness of unknown etiology in children. September 26, 2014. http://emergency.cdc.gov/han/han00370.asp. Accessed April 8, 2015.
11. Potter PC. Current guidelines for the management of asthma in young children. Allergy Asthma Immunol Res. 2010;2(1):1-13. www.ncbi.nlm.nih.gov/pmc/articles/PMC2831604. Accessed April 12, 2015.
12. CDC. Antibiotic resistance threats in the United States, 2013. www.cdc.gov/drugresistance/threat-report-2013. Accessed April 12, 2015.
13. Acetaminophen dosage for infants and children. Tylenol. www.tylenol.com/children-infants/safety/dosage-charts. Accessed April 12, 2015.
14. Consumer Healthcare Products Association. Briefing Book: Joint Meeting of the Nonprescription Drugs Advisory Committee and Pediatric Advisory Committee. May 17-18, 2011. www.chpa.org/workarea/downloadasset.aspx?id=1349. Accessed April 13, 2015.
15. Clinical Pharmacology [online database]. Tampa, FL: Gold Standard, Inc; 2013. www.clinicalpharmacology.com. Accessed April 13, 2015.
16. OCEAN for Kids Saline Nasal Spray. Valeant Consumer Products. www.oceannasalcare.com/saline-nasal-products/kids-saline-nasal-spray#how-it-works. Accessed April 13, 2015.
17. CDC. What parents need to know about Enterovirus D68. www.cdc.gov/features/evd68. Accessed April 13, 2015.
To comment on this article, contact rdavidson@uspharmacist.com.





