US Pharm. 2015;40(12):28-32.
ABSTRACT: Guidelines from the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition define the difference between gastroesophageal reflux (GER) and gastroesophageal reflux disease (GERD). Accurate distinction is important in the pediatric population in order for clinicians to determine which patients should be conservatively treated with lifestyle modifications or receive pharmacologic management. The use of acid-suppressant therapy (histamine2-receptor antagonists and proton pump inhibitors) is recommended in pediatric patients with persistent symptoms of GERD.
Gastroesophageal reflux (GER) is the passage of gastric contents into the esophagus, which is associated with transient relaxations of the lower esophageal sphincter (FIGURE 1).1,2 This occurs in more than two-thirds of otherwise healthy infants and can happen several times a day in healthy infants, children, and adults. In healthy adults, GER occurs post-prandial, lasts <3 minutes, and causes few or no symptoms.3 However, less is known regarding the physiology of GER in pediatrics, but the most visible symptom is regurgitation or spitting up, which occurs daily in 50% of all infants. Gastroesophageal reflux disease (GERD) is defined as troublesome symptoms or complications associated with GER.2
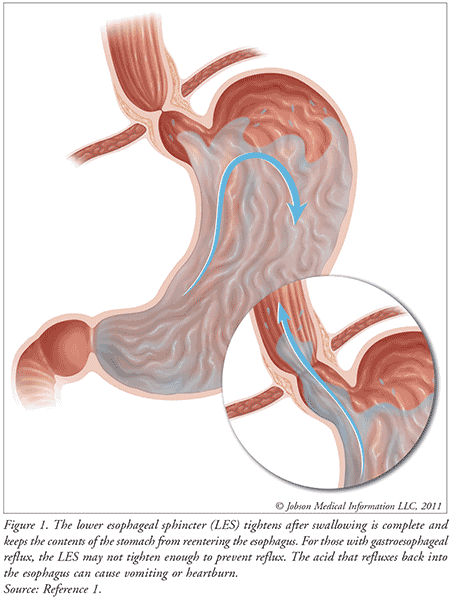
Symptoms or conditions associated with GERD are classified into two categories, esophageal or extra-esophageal.2 Esophageal conditions include vomiting, poor weight gain, abdominal or substernal/retrosternal pain, esophagitis, and dysphagia. Extra-esophageal conditions that are established manifestations of GERD include respiratory symptoms such as cough, laryngitis, and wheezing in infancy.4
EPIDEMIOLOGY
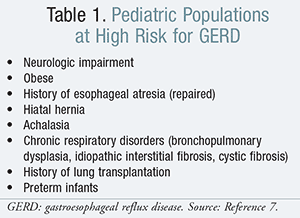
The prevalence of GERD in all age groups worldwide is increasing. In comparison to Eastern Asia, where the prevalence is 8.5%,5 the Western European and North American population have a higher prevalence of 10% to 20%.6 Those in the pediatric population considered to be at high risk for GERD are listed in TABLE 1. Pediatric patients with conditions that render them at high risk for GERD may be more prone to developing complications of severe GERD compared to healthy children.7
CLINICAL PRESENTATION
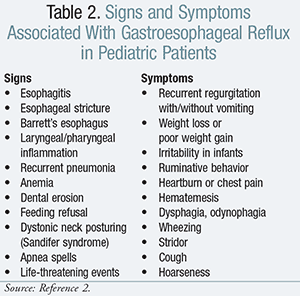
The signs and symptoms attributed to pediatric patients presenting with GER are shown in TABLE 2.2
Infants (aged <1 year) commonly present with regurgitation of food, vomiting associated with irritability, anorexia or feeding refusal, poor weight gain, dysphagia, and arching of the back during feedings.7 Regurgitation may occur postprandial or with delays of up to 1 to 2 hours.8 In children ages 1 to 5 years, common symptoms of GERD include regurgitation, vomiting, abdominal pain, anorexia, and feeding refusal. Older children and adolescents resemble adults with their clinical presentation of GERD. These children and adolescents complain of heartburn, epigastric pain, chest pain, nocturnal pain, dysphagia, and sour burps.7
Other characteristics to consider in a vomiting child are anatomic anomalies, protein allergy, or inborn metabolic disorders, which are rare. GER is a complicating factor in asthma associated with microaspiration, which leads to reflex bronchoconstriction. Cough, stridor, and pharyngitis have also been linked to GER. In addition, rumination is commonly observed in patients with developmental delay.8
The incidence of GERD is reported to be lower in breastfed infants than in formula-fed infants. In general, GERD may cause symptoms without necessarily interfering with growth. However, those children with clinically significant GERD or diagnosed with esophagitis may develop an aversion to food due to the stimulus-response associated as a result of pain with eating.7
DIAGNOSIS
The commonly used diagnostic methods to evaluate pediatric patients with GERD symptoms are upper gastrointestinal (GI) tract contrast radiography, esophageal pH and/or impedance monitoring, and upper endoscopy with esophageal biopsy.7
The upper GI tract contrast radiography allows the practitioner to delineate anatomy and document any presence of a motility disorder. This diagnostic method involves obtaining a series of fluoroscopic images of swallowed barium until the ligament of Treitz is visualized. This is not the preferred diagnostic method to diagnose GER or GERD since the test is too brief in duration to adequately rule out the occurrence of pathologic reflux. However, the upper GI tract series is helpful in evaluating vomiting to screen for anatomic abnormalities of the upper GI tract.2
The esophageal pH monitoring method is used to quantify the frequency and duration of esophageal acid exposure during a study period. Despite allowing the practitioner to associate a temporal relationship between a symptom and acid reflux and to evaluate the efficacy of pharmacologic therapy on acid suppression, there is evidence suggesting poor reproducibility of pH testing.9
Multiple intraluminal impedance detects the movement of both acidic and nonacidic fluids, solids, and air in the esophagus, which allows a more detailed picture of esophageal events compared to pH monitoring.9
The upper endoscopy diagnostic method of GERD allows direct visualization of the esophageal mucosa in order to assess the presence and severity of injury from the reflux of gastric contents into the esophagus.10 Upper endoscopy is indicated for patients with GERD who fail to respond to pharmacologic therapy or as part of initial management if the patient has symptoms of poor weight gain, unexplained anemia or fecal occult blood, recurrent pneumonia, or hematemesis. If the upper endoscopy diagnostic method is used in assessing GERD in pediatric patients, the benefits must be weighed against procedural and sedation risks.11 Esophageal biopsy is useful in evaluating conditions that may mimic symptoms of GERD, such as Crohn’s disease, Barrett’s esophagus, infectious esophagitis, or eosinophilic esophagitis. Esophageal biopsies should be performed during endoscopy because endoscopic findings correlate poorly with histologic testing in infants and children.2
CURRENT MANAGEMENT
Nonpharmacologic Management
Treating infants with GERD may involve a combination of feeding and positioning changes. Maternal diet may require modification since milk protein allergy can cause a clinical presentation that mimics GERD in infants. For breastfed infants, a 2- to 4-week trial of restricting the intake of milk and egg in the mother’s diet is recommended. Formula with an extensively hydrolyzed protein or amino acid–based formula may be appropriate in formula-fed infants. Another feeding management strategy is to use thickened feedings by either adding 1 tablespoon of dry rice cereal per 1 oz of formula or change to commercially thickened formulas for full-term infants not intolerant to cow milk protein.2
Infants with GERD may also benefit from positioning changes by keeping them in an upright position or placing them prone. However, the prone position is only acceptable if the infant is observed and awake due to the risk of sudden infant death syndrome (SIDS). The prone position is more beneficial to children older than 1 year since the risk of SIDS declines in older age groups.2
In older children and adolescents, it is recommended to avoid symptom triggers such as caffeine, chocolate, alcohol, spicy foods, fruit juices, tomato and citrus products, and peppermint.2,8 In addition, there is evidence from studies showing that chewing of sugarless gum postprandial may help to decrease reflux episodes.12-14
Pharmacologic Management
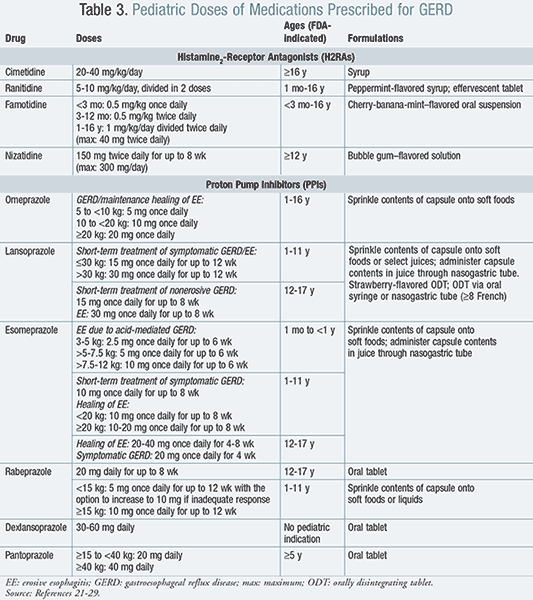
The two major classes of pharmacologic agents to treat GERD are acid suppressants and prokinetic agents. The main classes of acid suppressants include antacids, histamine2-receptor antagonists (H2RAs), and proton pump inhibitors (PPIs) (TABLE 3).7
Antacids: Antacids reduce heartburn by directly buffering gastric acid in the esophagus or stomach. This mechanism allows mucosal healing of the esophagus. However, there is limited evidence showing symptom relief in infants and children. Caution should be exercised if considering the use of antacids in treating the pediatric population. Several studies link the use of antacids that contain aluminum to aluminum toxicity and complications in children.15-17 Children who receive calcium-containing preparations have experienced milk-alkali syndrome, a triad of hypercalcemia, alkalosis, and renal failure. According to guidelines from the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition, chronic antacid therapy is generally not recommended for the treatment of GERD in pediatrics. In addition, the safety and efficacy of surface protective agents, such as alginates or sucralfate, have not been adequately studied in the pediatric population.7
H2RAs: The mechanism of action of H2RAs is that they work to decrease acid secretion by inhibiting H2 receptors on gastric parietal cells. The results of randomized, placebo-controlled, pediatric clinical trials have shown that cimetidine and nizatidine are superior to placebo for the treatment of symptoms and healing of esophageal mucosa in children.2,18,19 Randomized, placebo-controlled, pediatric clinical trials of famotidine or ranitidine have not demonstrated efficacy in children, but expert opinion is that these agents are just as effective as cimetidine and nizatidine.2 It has been determined from pharmacokinetic studies in 4- to 11-year-old children that peak plasma ranitidine concentration occurs 2.5 hours after dosing with a half-life of 2 hours. Gastric pH begins to increase within 30 minutes of administering the H2RA, and the effect lasts for about 6 hours.2,7
It is prudent that children be monitored for tachyphylaxis, which may develop within 6 weeks of treatment initiation, therefore limiting its long-term use. In addition, H2RAs are linked to liver disease and, specifically, cimetidine has been linked to an increased risk of gynecomastia; these may be similar to the effects of other H2RAs. H2RAs may also cause other adverse events similar to persistent symptoms of GERD. These side effects include irritability, head banging, headache, or somnolence. Caution must be used to determine the cause of these symptoms, so the doses of H2RAs are not inappropriately increased.2
PPIs: The most potent class of drugs to demonstrate superior efficacy compared to H2RAs are PPIs. PPIs inhibit the H+, K+-ATPase in the gastric parietal cell canaliculus, thereby decreasing acid secretion. In comparison to H2RAs, PPIs can maintain gastric pH >4 and inhibit meal-induced acid secretion for a longer period of time. Acid suppression capabilities from PPIs also do not diminish with chronic use. PPIs provide faster healing rates for erosive esophagitis than H2RAs. Administering times of PPIs is crucial for maximum efficacy for the patient. Pharmacists should counsel and educate the patients or caretakers to administer PPIs approximately 30 minutes before meals.7
In general, PPIs are safe and well tolerated with few adverse effects. It has been reported that headaches, diarrhea, constipation, and nausea have occurred in 14% of older children and adults taking PPIs.7 A reduction in irritability from PPIs over placebo was not demonstrated in placebo-controlled trials in infants.20
Other: There is insufficient evidence to support the use of any prokinetic agent, including metoclopramide, for the treatment of GERD in the pediatric population.18 Surgical management such as fundoplication or total esophagogastric dissociation are reserved for those patients with intractable symptoms, failed pharmacologic treatment, or who are experiencing life-threatening complications of GERD.2
CONCLUSION
GER is a normal physiologic process occurring in the healthy pediatric population and adults alike. GERD symptoms may occur as a complication associated with GER, and it is important for clinicians to accurately diagnose and assess how best to manage the patient to improve symptoms and facilitate healing of the esophagus. Pediatric patients with GER who experience uncomplicated recurrent regurgitation should be managed conservatively with minimal testing and lifestyle modifications. Clinicians should carefully monitor pediatric patients with GERD to ensure their symptoms are improving with medication management. It is also important to avoid any unnecessary diagnostic procedures or pharmacologic therapy as best practice in the pediatric population.
REFERENCES
1. Gastroesophageal reflux disease (GERD)/heartburn in children. Johns Hopkins Medicine. www.hopkinsmedicine.org/healthlibrary/conditions/pediatrics/gastroesophageal_reflux_disease_gerdheartburn_in_children_90,P01994/. Accessed July 24, 2015.
2. Vandenplas Y, Rudolph CD, Di Lorenzo C, et al; North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. Pediatric gastroesophageal reflux clinical practice guidelines: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN). J Pediatr Gastroenterol Nutr. 2009;49(4):498-547.
3. Shay S, Tutuian R, Sifrim D, et al. Twenty-four hour ambulatory simultaneous impedance and pH monitoring: a multicenter report of normal values from 60 healthy volunteers. Am J Gastroenterol. 2004;99(6):1037-1043.
4. Sheikh S, Goldsmith LJ, Howell L, et al. Lung function in infants with wheezing and gastroesophageal reflux. Pediatr Pulmonol. 1999;27(4): 236-241.
5. Jung HK. Epidemiology of gastroesophageal reflux disease in Asia: a systematic review. J Neurogasteroenterol Motil. 2011;17(1):14-27.
6. Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastero-oesophageal reflux disease: a systematic review. Gut. 2005:54(5):710-717.
7. Lightdale J, Gremse D. Gastroesophageal reflux: management guidance for the pediatrician. Pediatrics. 2013;131(5):e1684-e1695. http://pediatrics.aappublications.org/content/131/5/e1684.full. Accessed July 24, 2015.
8. Schwarz, S, Hebra, A. Pediatric gastroesophageal reflux. Medscape. http://emedicine.medscape.com/article/930029-overview. Accessed July 24, 2015.
9. Rosen R, Lord C, Nurko S. The sensitivity of multichannel intraluminal impedance and the pH probe in the evaluation of gastroesophageal reflux in children. Clin Gastroenteral Hepatol. 2006;4(2):167-172.
10. Hassall E. Decisions in diagnosing and managing chronic gastroesophageal reflux disease in children. J Pediatr. 2005;146(suppl 3):S3-S12.
11. Thakkar K, El-Serag HB, Mattek N, Gilger MA. Complications of pediatric EGD: a 4-year experience in PEDS-CORI. Gastrointest Endosc. 2007;65(2):213-221.
12. Avidan B, Sonnenberg A, Schnell TG, Sontag SJ. Walking and chewing reduce postprandial acid reflux. Aliment Pharmacol Ther. 2001;15(2):151-155.
13. Moazzez R, Bartlett D, Anggiansah A. The effect of chewing sugar-free gum on gastro-esophageal reflux. J Dent Res. 2005;84(11):1062-1065.
14. Smoak BR, Koufman JA. Effects of gum chewing on pharyngeal and esophageal pH. Ann Otol Rhinol Laryngol. 2001;110(12):1117-1119.
15. Sedman A. Aluminum toxicity in childhood. Pediatr Nephrol. 1992;6(4):383-393.
16. Tsou VM, Young RM, Hart MH, Vanderhoof JA. Elevated plasma aluminum levels in normal infants receiving antacids containing aluminum. Pediatrics. 1991;87(2):148-151.
17. American Academy of Pediatrics, Committee on Nutrition. Aluminum toxicity in infants and children. Pediatrics. 1996;97(3):413-416.
18. Gremse DA. GERD in the pediatric patient: management considerations. MedGenMed. 2004;6(2):13.
19. Simeone D, Caria MC, Miele E, Staiano A. Treatment of childhood peptic esophagitis: a double-blind placebo-controlled trial of nizatidine. J Pediatr Gastroenterol Nutr. 1997;25(1):51-55.
20. Moore DJ, Tao BS, Lines DR, et al. Double-blind placebo-controlled trial of omeprazole in irritable infants with gastroesophageal reflux. J Pediatr. 2003;143(2):219-223.
21. Tagamet (cimetidine) product information. Boronia, Victoria: GlaxoSmithKline; March 2008. www.gsk.com.au/resources.ashx/prescriptionmedicinesproductschilddataproinfo/459/FileName/6983316077750639F808B4C371EE0AA6/PI_Tagamet.pdf. Accessed November 7, 2015.
22. Zantac (ranitidine) tablets package insert. Research Triangle Park, NC: GlaxoSmithKline; February 2015. www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Zantac_Oral/pdf/ZANTAC-ORAL.PDF. Accessed November 7, 2015.
23. DiMarino MC. Drug treatment of gastric acidity. Merck Manual Professional Version. www.merckmanuals.com/professional/gastrointestinal-disorders/gastritis-and-peptic-ulcer-disease/drug-treatment-of-gastric-acidity#section_7. Accessed November 7, 2015.
24. Axid (nizatidine) prescribing information. Braintree, MA: Braintree Laboratories, Inc.; January 2012. http://druginserts.com/lib/rx/meds/axid/page/3/#s46. Accessed November 7, 2015.
25. Prilosec (omeprazole) delayed-release capsules package insert. Wilmington, DE: AstraZeneca; March 2014. www1.astrazeneca-us.com/pi/Prilosec.pdf. Accessed November 7, 2015.
26. Prevacid (lansoprazole) delayed-release capsules and SoluTab orally disintegrating tablets package insert. Deerfield, IL: Takeda Pharmaceuticals America, Inc; December 2014. http://general.takedapharm.com/content/file.aspx?filetypecode=PREVACIDPI&cacheRandomizer=16442e0b-ed2e-4398-bba9-fd36259e03c8. Accessed November 7, 2015.
27. Nexium (esomeprazole) delayed-release capsules package insert. Wilmington, DE: AstraZeneca; December 2014. www.azpicentral.com/nexium/nexium.pdf#page=1. Accessed November 7, 2015.
28. Aciphex Sprinkle (rabeprazole sodium) delayed-release capsules package insert. Charlotte, NC: FSC Pediatrics; December 2014. www.aciphexsprinkle.com/wp-content/uploads/2015/01/20141219-Aciphex-PI-and-MedGuide-clean-December-2014.pdf. Accessed November 4, 2015.
29. Protonix (pantoprazole sodium) delayed-release tablets package insert. Philadelphia, PA: Wyeth Pharmaceuticals (Pfizer); December 2014. http://labeling.pfizer.com/ShowLabeling.aspx?id=135. Accessed November 4, 2015.
To comment on this article, contact rdavidson@uspharmacist.com.





