US Pharm. 2015;40(3):HS17-HS20.
ABSTRACT: In 2011, the FDA approved a long-acting, liposomal, sustained-release formulation of bupivacaine (Exparel) indicated for postoperative pain in adults. As many hospitals and health systems grapple with increasing drug costs juxtaposed with pressures to reduce operating expenditures, the use of this new agent must be carefully evaluated. Liposomal bupivacaine is an encouraging step forward in drug delivery and formulation design. While the drug appears to be relatively well tolerated, a lack of head-to-head studies and clinical trials utilizing the drug in only specific surgical situations limits the applicability of available research to clinical practice.
Liposomal drug technology is an important advancement in drug delivery, first described in the 1950s, which allows the safe and efficacious delivery of drug particles. A liposome is composed of an aqueous core with a lipid outer layer, and it can be a carrier for lipophilic or hydrophilic drug compounds.1 Liposomal bupivacaine (Exparel) uses DepoFoam technology, a type of liposomal drug delivery that releases the bupivacaine over an extended period of time without affecting the active ingredient itself. DepoFoam technology is also used in two other FDA approved drugs, DepoCyt (cytarabine) and DepoDur (morphine), extended-release products for use in lymphomatous meningitis and postsurgical analgesia, respectively.2
Liposomal bupivacaine is indicated for postsurgical analgesia via single-dose infiltration injection into the surgical site.3 A single dose provides analgesia for up to 72 hours. Liposomal bupivacaine has enjoyed commercial success since entering the marketplace in 2011, as the product has consistently increased sales every quarter.4,5 Additionally, the number of purchasers is increasing, indicating more widespread utilization of the product across the healthcare continuum.
Clinical Need
Currently accepted treatment for postsurgical pain management includes a multimodal approach consisting of several therapies, as no single agent adequately treats all types of pain. One treatment option is administration of anesthetics such as bupivacaine or lidocaine at the incision site. Conventional anesthetics have a relatively short duration of efficacy when administered alone and may be combined with epinephrine to promote a longer period of analgesia. However, even with the addition of epinephrine, these agents wear off relatively quickly (8-12 hours),6 necessitating additional medications for adequate pain control.
Another method for increasing the analgesic duration of conventional local anesthetics is the use of elastomeric pain pumps. These pumps slowly infiltrate anesthetic at the incision site through a small catheter. This treatment option requires patients to wear the device for 3 to 5 days, which can limit ability to resume normal activities, and patients must undergo eventual catheter removal.
A common treatment used for pain relief in the postsurgical period is opioids.7 Opioid medications are relatively inexpensive, some formulations have a longer duration of action than local anesthetics, and they are easily administered orally or via existing parenteral access. Unfortunately, this class of medications is associated with a multitude of adverse effects that may be dangerous in patients who have recently undergone surgery, including sedation, dizziness, nausea, and constipation.7
Other analgesic options include nonsteroidal anti-inflammatory drugs (NSAIDs), which may provide an insufficient level of analgesia and can have adverse effects that include gastrointestinal (GI) symptoms and blood-thinning properties that may be undesirable in the postsurgical population.8
Liposomal bupivacaine is intended to fill a niche as a long-acting analgesic that may be infiltrated in the perioperative or postsurgical period and provide analgesia for a longer period than conventional anesthetics alone or with epinephrine. Additionally, the drug does not require the use of expensive and cumbersome elastomeric pumps and spares opioid medications with potentially detrimental side effects.7
Clinical Efficacy
Three phase III studies and 13 phase I and II studies were provided to the FDA as a part of the liposomal bupivacaine New Drug Application (NDA).9-11 Two of the phase III studies, which have been published in peer-reviewed journals, compared liposomal bupivacaine to placebo.9,10 The third phase III study, SIMPLE 312, compared liposomal bupivacaine to bupivacaine 100 mg with epinephrine (1:200,000).11 All three of these studies compared liposomal bupivacaine using the primary endpoint of AUC-NRS (area under the curve of pain scores numeric rating scale) of 0 to 10, with several secondary endpoints. It should be noted that two of these studies evaluated a 300-mg dose of liposomal bupivacaine,9,11 which is larger than the 266-mg maximum approved by the FDA.3 These studies are summarized in TABLE 1.9-11
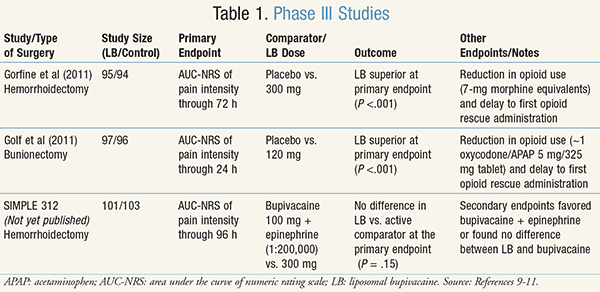
In the first study, Gorfine et al compared 300 mg of liposomal bupivacaine to placebo in patients undergoing hemorrhoidectomy.9 It was a multicenter, double-blind, randomized, phase III study enrolling 189 patients across 13 centers in three countries. Enrolled patients did not use any analgesics (opioids, NSAIDs, or acetaminophen [APAP]) 72 hours prior to surgery, and were given morphine 10 mg IM as opioid rescue after surgery and liposomal bupivacaine or placebo administration. The authors found a statistically significant reduction in cumulative pain score at 72 hours (P < .001) with liposomal bupivacaine compared to placebo, which was largely driven by differences in pain scores in the first 24 hours after surgery. The FDA reviewer noted in his review that “analgesia derived from [liposomal bupivacaine] does not differ from placebo, at least not in a clinically meaningful way, beyond 24 hours.”11
In the second study, Golf et al compared 120-mg liposomal bupivacaine to placebo in patients undergoing bunionectomy.10 It was a multicenter, double-blind, randomized, phase III trial enrolling 193 patients. Enrolled patients could not be chronic users of pain medications (defined as opioid use >14 days in the last 3 months or nonopioid use >5 times per week) or have used NSAIDs within 72 hours of surgery or APAP within 24 hours of surgery. Patients were able to use 1 to 2 oxycodone/APAP 5 mg/325 mg tablets every 4 to 6 hours as needed for pain after surgery and were eligible to receive a single 15- to 30-mg IV dose of ketorolac if pain could not be controlled by oxycodone/APAP alone. This study found a statistically significant reduction in cumulative pain score at 24 hours (P <.001) with liposomal bupivacaine compared to placebo. No difference in pain scores was observed at 48 hours or beyond.10
The third study, SIMPLE 312, which has not yet been published in a peer-reviewed journal, compared 300 mg of liposomal bupivacaine to 100 mg of bupivacaine with epinephrine (1:200,000) in patients undergoing hemorrhoidectomy.11 This phase III multicenter, double-blind, randomized trial enrolled 204 patients. Patients were excluded for using any long-acting opioid within 3 days of study enrollment or a short-acting opioid within 24 hours of enrollment. All patients received a dose of IV ketorolac (or equivalent) at the end of surgery and received APAP 1,000 mg three times daily for 4 days as soon as the patient was able to tolerate oral medications. The study failed to find a statistically significant difference in the primary endpoint of AUC-NRS at 96 hours (P = .15). Overall, it failed to find a statistically or clinically significant difference in pain scores at any time endpoint, including at 24 hours.11
All three of the phase III studies presented to the FDA had dozens of secondary endpoints, which were statistically compared, but there were no prior adjustments to the statistical analysis plans to account for multiple endpoints.11 Some of these secondary endpoints included time to opioid rescue, total use of opioids, and provider and patient satisfaction. The two studies9,10 that compared liposomal bupivacaine to placebo overall found all secondary endpoints favored liposomal bupivacaine, including a reduction in total opioid use as well as delay to first opioid administration. Golf et al found that 28% of patients treated with liposomal bupivacaine did not require any opioids in the postsurgical period compared with 10% of patients treated with placebo.10 Gorfine et al similarly found a greater percentage of patients treated with liposomal bupivacaine who did not require any opioids.9 These authors concluded that “reduction in opioid consumption may result in fewer opioid-related AEs [adverse events], faster ambulation, and shorter duration of postsurgical hospital stays.” The authors recommended further studies to confirm these observations.9
Many other safety and efficacy studies with liposomal bupivacaine have been conducted and published in the literature for a variety of different surgeries including hernia repair, total knee arthroplasty, breast augmentation, and colectomy.14 These studies have looked at several different endpoints including opioid use, wound healing, hospital length of stay, and total cost of hospital stay. Liposomal bupivacaine has been linked to a reduction in postoperative opioid use,9,10 which may contribute to fewer opioid-related adverse events, but this has yet to be confirmed in randomized, controlled trials. Similarly, impaired wound healing has been associated with inadequate pain control, which may be improved with liposomal bupivacaine; however, a review of 10 studies by Baxter et al found no statistically or clinically significant differences in improved wound healing with liposomal bupivacaine.12
Clinical Safety
The tolerability and safety of liposomal bupivacaine has been established in patients >18 years of age.3 The most commonly reported adverse effects, occurring with an incidence of >10%, include nausea, constipation, and vomiting. Other adverse effects, occurring with an incidence of >2%, include dizziness, peripheral edema, hypotension, tachycardia, somnolence, and headache. Serious adverse events, including seizures and cardiac toxicity/cardiac arrest, can occur if liposomal bupivacaine is inadvertently administered IV or in overdose.3
Liposomal bupivacaine and other amide local anesthetics are metabolized by the liver and therefore should be used with caution in patients with hepatic dysfunction.3 The package insert states that patients with severe hepatic impairment “are at a greater risk of developing toxic plasma concentrations,” but does not recommend any dose adjustment or avoiding use.3 The risk versus benefits for patients with severe hepatic impairment must be weighed by the administering clinician.
Bupivacaine toxicity had previously been reported when plasma levels exceed 1 mcg/mL; however, pharmacokinetic data with liposomal bupivacaine evaluated patients with plasma levels >100 times this limit with no adverse effects.11 The package insert advises that any other amide local anesthetics, including any lidocaine or bupivacaine product, should not be administered within 96 hours of liposomal bupivacaine due to risk of elevated plasma levels.3 A pharmacokinetic study in an animal model found that the plasma concentrations of both lidocaine and liposomal bupivacaine were increased after coadministration.14 Although liposomal bupivacaine results in detectable plasma levels for 96 hours, these levels have not been correlated with local efficacy.3
Overall, liposomal bupivacaine has been well tolerated with adverse effects similar to those of bupivacaine with or without epinephrine.3,11 Please refer to TABLE 2 for a product summary.3

Formulary Considerations
Liposomal bupivacaine is available as a single-source brand name product, Exparel.3 It is sold as a 20-mL single-dose 13.3 mg/mL liposomal suspension. Exparel should be refrigerated but may be stored at room temperature for up to 30 days in sealed, unopened vials. Vials should be inverted multiple times to resuspend particles immediately prior to withdrawal from the vial, and diluted suspensions should be used within 4 hours of preparation in a syringe.3
The average wholesale price (AWP) of one 20-mL Exparel vial is $359.99.15 Compared to the $2 to $8 AWP of a vial of standard bupivacaine or bupivacaine with epinephrine,16 this is a substantial increase in therapy cost. For illustration purposes, a hospital performing 5,000 annual surgeries with a 10% utilization of liposomal bupivacaine in place of conventional bupivacaine could expect increased drug expenditures of up to $179,000 annually. Centers performing more surgeries or with higher liposomal bupivacaine utilization rates could see even greater increases in spending. Under diagnosis-related group billing or other flat-rate reimbursement models, this additional drug cost would decrease hospital margins and might create additional budgetary constraints elsewhere.
While proponents of liposomal bupivacaine may cite decreased need for opioid medications or hospital length of stay and superior pain control with its use, there have been no head-to-head studies demonstrating clinically meaningful differences in these metrics with this drug over standard of care. Well-designed pain management algorithms and clinical monitoring programs may help to contain costs and aid in improving pain control by using less-expensive medications in the treatment of postoperative pain.
Liposomal bupivacaine may have a role for postoperative pain control in outpatient or ambulatory surgery patients who experience adverse effects from opioids or NSAIDs or in whom these drugs are contraindicated. In this setting, patients are not routinely monitored for long periods of time after surgery, and liposomal bupivacaine may provide long-lasting analgesia and promote ambulation while avoiding the negative effects of other pain medications.
To assess pharmacoeconomic implications with liposomal bupivacaine, Cohen conducted an open-label, single-center, sequential cohort analysis of 39 patients undergoing open colectomy.13 The study compared an opioid-based pain regimen of a patient-controlled analgesia pump with hydromorphone or morphine to a multimodal approach with one dose each of liposomal bupivacaine and IV ketorolac after surgery plus oral APAP and ibuprofen every 6 hours after surgery. Both groups were offered IV opioids and/or oral oxycodone/APAP for rescue analgesia. Overall, patients treated with liposomal bupivacaine consumed less opioids and had a shorter length of hospital stay (2 days vs. 4.9 days), with a reduction in average cost of hospitalization ($8,766 vs. $11,850). This small, single-center analysis will need to be confirmed in a larger, randomized study.13
In terms of safety, it should be noted that liposomal bupivacaine is milky white in appearance and looks similar to propofol, a drug also commonly administered in the operating room. As propofol is administered via IV, there is a risk for route errors if liposomal bupivacaine and propofol are confused. The Institute for Safe Medication Practices published a statement in 2012 advising hospitals to evaluate their current processes and provided best practices to reduce the risk of error.17 Although there have not been reports of administration errors with propofol and liposomal bupivacaine, this proactive approach was warranted, as confusion of propofol and liposomal bupivacaine could lead to toxic effects, including death. Increased use of liposomal bupivacaine may increase the chance of look-alike error occurrence.
Conclusion
The emergence of liposomal bupivacaine is an encouraging step forward in drug delivery and formulation design. The drug appears to be relatively well tolerated and may be as effective as conventional bupivacaine (with or without epinephrine) in certain populations. However, a lack of head-to-head studies with standard therapy and trials utilizing the drug in only specific surgical situations limits the applicability of available research to clinical practice. Furthermore, the increased cost of liposomal bupivacaine over generically available alternatives may prove to be a significant burden on hospitals and health systems. While the promises of improved pain scores, decreased length of stay, and superior pain control are appealing, available evidence is not sufficient to support the conclusion that these outcomes compare with currently used therapies. Additional head-to-head studies assessing clinical efficacy and cost justification are needed before widespread use of liposomal bupivacaine can be advised.
REFERENCES
1. Kraft JC, Freeling JP, Wang Z, Ho RJ. Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. J Pharm Sci. 2014;103:29-52.
2. Exparel. About DepoFoam. Pacira Pharmaceuticals, Inc. www.exparel.com/how-to-use/about-depofoam.shtml. Accessed February 19, 2015.
3. Exparel (liposomal bupivacaine) package insert. San Diego, CA: Pacira Pharmaceuticals, Inc; December 2014.
4. Pacira Pharmaceuticals, Inc. Reports first quarter EXPAREL revenue of $34.4 million and first quarter 2014 results. Press release. Marketwatch. May 1, 2014. www.marketwatch.com/story/pacira-pharmaceuticals-inc-reports-first-quarter-exparel-revenue-of-344-million-and-first-quarter-2014-results-2014-05-01. Accessed November 26, 2014.
5. Pacira Pharmaceuticals, Inc. Reports 2013 financial results: fourth quarter EXPAREL revenues increase 53 percent over third quarter. News release. February 25, 2014. http://investor.pacira.com/phoenix.zhtml?c=220759&p=irol-newsArticle&ID=1903060&highlight. Accessed November 26, 2014.
6. Skolnik A, Gan TJ. New formulations of bupivacaine for the treatment of postoperative pain: liposomal bupivacaine and SABER-Bupivacaine. Expert Opin Pharmacother. 2014;15:1535-1542.
7. Oderda G. Challenges in the management of acute postsurgical pain. Pharmacotherapy. 2012;32(9 pt 2):6S-11S.8. Soloman D. NSAIDs: therapeutic use and variability of response in adults. UpToDate. Waltham, MA: UptoDate; 2014. www.uptodate.com. Accessed December 24, 2014.
9. Gorfine SR, Onel E, Patou G, Krivokapic ZV. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trial. Dis Colon Rectum. 2011;54:1552-1559.
10. Golf M, Daniels SE, Onel E. A phase 3, randomized, placebo-controlled trial of DepoFoam bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Ther. 2011;28:776-788.
11. Simone A. NDA 022-496. Exparel (bupivacaine). Center for Drug Evaluation and Research clinical review. www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022496Orig1s000MedR.pdf. Accessed November 26, 2014.
12. Baxter R, Bramlett K, Onel E, Daniels S. Impact of local administration of liposome bupivacaine for postsurgical analgesia on wound healing: a review of data from ten prospective, controlled clinical studies. Clin Ther. 2013;35: 312-320.
13. Cohen SM. Extended pain relief trail utilizing infiltration of Exparel, a long-acting multivesicular liposome formulation of bupivacaine: a phase IV health economic trial in adult patients undergoing open colectomy. J Pain Res. 2012;5:567-572.
14. Noviasky J, Pierce DP, Whalen K, et al. Bupivacaine liposomal versus bupivacaine: comparative review. Hosp Pharm. 2014;49:539-543.
15. Liposomal bupivacaine: drug information. UpToDate. Waltham, MA: UptoDate; 2014. www.uptodate.com. Accessed December 24, 2014.
16. Bupivacaine: drug information. UpToDate. Waltham, MA: UptoDate; 2014. www.uptodate.com. Accessed December 24, 2014.
17. Potential for wrong route errors with Exparel (bupivacaine liposome injectable suspension). National Alert Network. March 20, 2012. www.ismp.org/NAN/files/NAN-20120318.pdf. Accessed December 24, 2014.






