US Pharm. 2015;40(5):52-55.
The new class of antipsychotic medications is called atypical antipsychotics, also known as second-generation antipsychotics (SGAs). “Atypical” means they work in a way that is significantly different from that of the previous class of antipsychotics (e.g., haloperidol, Thorazine), known as first-generation antipsychotics (FGAs). Antipsychotics originally were thought only to help people with psychosis. However, since their initial development, further research has indicated that this class of drugs could also be helpful in mood stabilizing. Thus, for someone with bipolar disorder, the mood swings will typically become less frequent and less intense.1
Pediatric Use
Atypical antipsychotics offer superior safety and similar efficacy compared with conventional agents in adults with psychotic disorders. For this reason, atypical antipsychotics have been increasingly used in children and adolescents. These drugs are not interchangeable—each has a unique pharmacologic profile and may differ considerably in terms of adverse effects. Five agents currently have FDA-approved indications for use in children and adolescents: risperidone, aripiprazole, olanzapine, paliperidone, and quetiapine. Despite their widespread use, atypical antipsychotics are not FDA-approved for children <5 years of age.1,2
The term pediatric patients includes children and adolescents. Children are further defined to be any patients aged 6 to 12 years and adolescents are 13 to 17 years. The use of antipsychotic drugs for pediatric patients with behavior problems doubled between 1999-2001 and 2007.1
Atypical antipsychotic use in pediatric patients has also been increased for indications that are not FDA-approved. The FDA-approved indications in pediatric patients for atypical antipsychotics are schizophrenia, bipolar I disorder (manic or mixed), and irritability with autistic disorders.1 Clinicians may first suggest a different type of drug, psycho-therapy, or some other type of nonpharmacologic treatment before suggesting an antipsychotic in a young child.
Indications
FGAs, also known as neuroleptics, were initially shown to provide efficacious treatment for schizophrenia, acute and chronic psychosis, postpartum psychosis, acute mania and hypomania, and management of neuropsychiatric symptoms of dementia and bipolar disorders in adults. However, these drugs cause extrapyramidal symptoms (EPS), including rigidity, bradykinesia, tremor, and restlessness. They also frequently lead to tardive dyskinesia and hyperkinetic, involuntary movements most readily observed in the face and extremities.2
Atypical antipsychotics or SGAs are used for symptomatic treatment of pervasive developmental problems such as autism, Asperger syndrome and other autism spectrum disorders, Tourette syndrome, attention-deficit/hyperactivity disorder (ADHD), and disruptive behavior disorders such as oppositional defiant disorder and conduct disorders. In terms of adverse effects, they have generally lower risks of EPS and tardive dyskinesia compared to FGAs. However, these medications cause higher rates of weight gain and metabolic disorders (e.g., hypertension, hyperglycemia, high cholesterol). The adverse effects of SGAs vary for each individual drug.2,3
Mechanisms of Action
The common activity of both of these groups appears to be postsynaptic blockade of brain dopamine D2 receptors. An exception is aripiprazole, as it is a D2 partial agonist. Several major research studies support the role of these receptors in the activity of antipsychotics, noting a correlation between receptor binding and pharmacologic potency. For all these medications to show their effects, a 60% to 65% D2 receptor occupancy is a consistent requirement. This has been reported by functional imaging studies.3
SGAs differ from the older class in that serotonin 5-HT2 receptor binding can exceed their affinity for dopamine D2 receptors. The 5-HT2 activity has been suggested for lower risk of EPS. In addition, the drugs differ as to how tightly they bind to D2 receptors. Two of the atypical antipsychotics, clozapine and quetiapine, bind loosely and turn over after a few minutes, in contrast to all other antipsychotics, which bind tightly and turn over after several hours. The exceptionally low risk of EPS with clozapine and quetiapine may be related to that binding characteristic.3
All atypical antipsychotics differ in other aspects of their pharmacology, making each medication unique in its side-effect profile, dosing regimen, rate of absorption, rate and way of elimination, and drug-drug interactions.
Adverse Reactions and Risk Factors
Adverse effects associated with SGAs include diabetes, weight gain, hyperlipidemia, hyperprolactinemia, and sudden death. Some side effects occur at a rate and severity that varies across individual SGAs. These will be described under each individual drug.3,4
The healthcare team, patient, and parents should be made aware of the risks of taking an atypical antipsychotic prior to initiating therapy. Because these medications are associated with significant weight gain and metabolic changes, a baseline for weight should be established and the patient routinely monitored for weight and metabolic changes. Specific recommendations for monitoring are provided in the prescribing information for each drug. Common adverse reactions include sedation, orthostatic hypotension, tachycardia, QT prolongation, menstrual problems, blurred vision, dry skin, and sun sensitivity.4
Safety and efficacy in pediatric patients have not been established for asenapine, clozapine, iloperidone, lurasidone, and ziprasidone. Manufacturers of atypical antipsychotics warn against their use in pediatric patients with a history of seizure disorders, since these medications may lower seizure threshold.4
Two atypical antipsychotics, aripiprazole and quetiapine, are FDA-approved for the treat-ment of depressive episodes in bipolar I disorders or as adjunctive treatment for major depressive disorders in adults, but not in pediatric patients. Therefore, if prescribed for this age group, the risk of suicidality may still be present.4
Individual Medications
Pharmacology, recommended dosing (TABLE 1), and side-effect profiles unique to each FDA-approved atypical antipsychotic for children and adolescents are described in the following sections.5-10
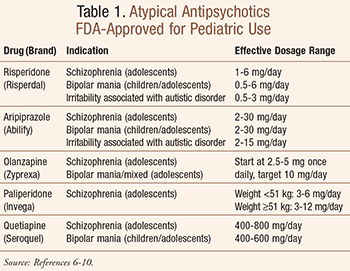
Risperidone: This drug is available as tablets, rapid-dissolving tablets, liquid, and injectable forms.5,6 The pharmacokinetics of the oral formulations are similar, with rapid absorption and a 20-hour elimination half-life. A depot injectable form of risperidone is also available (Risperdal Consta). The serum level of this drug is decreased by carbamazepine and increased by fluoxetine and ketoconazole. This drug is dosed once daily, 0.25 to 6 mg/day. Doses above 6 mg/day cause EPS. Renal dysfunction reduces elimination of risperidone by 60%.5,6
Weight gain is a documented adverse effect of risperidone in adolescents diagnosed with schizophrenia.5,6 In one open-label study, 14% of the patients experienced an average weight gain of 9 kg (about 20 lb) during 8 months of risperidone treatment, with much of the weight gain occurring during the first 6 months of treatment. When a patient is taking risperidone, monitoring weight gain and related factors against normal development patterns is recom-mended. Other side effects include hypotension, akathisia, and prolactin elevation. Risperidone should not be used by patients aged >17 years who have been diagnosed with irritability with autistic disorders. This drug and aripiprazole have been used more than other agents for irritability with autistic disorders in children aged 5 to 17 years.5,6
Aripiprazole: This drug is unique among the SGAs in its pharma-cology and pharmacokinetics, although it is similar in clinical efficacy.7 Aripiprazole acts as a partial agonist at dopamine D2 receptors, a partial agonist at serotonin 5-HT1a receptors, but an antagonist at 5-HT2a and alpha1-adrenergic receptors. It is available as tablets, fast-dissolving tablets, and sterile solution for IM injection. The oral preparations act slowly, but the injectable form shows fast clinical response within 45 minutes. The long elimination half-life gives a steady serum level throughout the day. Many clinicians make dosage adjustment based on the patient’s clinical response. Doses of 2 to 30 mg daily have been adequate for most patients.7
Adverse effects of aripiprazole include headache, insomnia, tremor, and constipation.7 The use of this medication with other antipsychotics is not recommended because the combination of a partial agonist and antagonist leads to unpredictable levels of receptor activity. The incidence of events related to EPS in adults diagnosed with schizophrenia who were being treated with aripiprazole mono-therapy was 13% versus 12% for placebo. In pediatric patients aged 13-17 years who were treated with aripiprazole, the percentage of EPS-related events was 25% versus 7% for placebo.7
Aripiprazole is not indicated as monotherapy for major depressive disorder in any population. This medication can increase the risk of suicidal thinking in children and adolescents.7
Olanzapine: This drug is available as tablets, fast-dissolving tablets, and short- and long-acting inject-able forms such as olanzapine pamoate.8 It is given 2.5 to 20 mg once daily for acute agitation. Olanzapine is rapidly oxidized in air, and coated tablets, if cut, should be used immediately. It has significant activity at hista-minic and muscarinic receptors. Cigarette smoking decreases levels of this drug, and clinicians should be aware of this during dosage adjustment. Olanzapine causes less somnolence than alternative drugs commonly used for agitation. It is recommended that olanzapine and diazepam injectables not to be combined due to possible cardiovascular risk.8
Adolescents who take olanzapine have an increased potential for weight gain, hyperglycemia, and hyperlipidemia compared with adult patients who take this agent. No dosage adjustment is necessary with renal and hepatic impairment. The prescribing information for olanzapine states: “Clinicians should consider the potential long-term risks when prescribing to adolescents, and in many cases this may lead them to consider prescribing other drugs first in adolescents.”8
Paliperidone: This drug is available in an osmotic delivery capsule to be swallowed whole, not crushed or chewed, and requires several days to achieve steady-state kinetics.9 Its bioavailability is increased by 50% when taken with a high-calorie meal. It is mainly excreted unchanged in the urine. The dosage is 3 to 12 mg once daily.9
Metabolic changes such as weight gain have been associated with paliperidone, but these effects are considered mild-to-moderate.9 It is recommended that the dose of paliperidone be reduced about 50% if combined with fluoxetine. A baseline hemoglobin A1C level, blood glucose level, and lipid panel should be obtained, and periodic monitoring of a patient’s weight is recommended. Other adverse effects include dizziness, orthostatic hypotension, tachycardia, and dry mouth. Paliperidone causes relatively few EPS and mild QT prolongation, but not to a degree that cardiac monitoring is recommended.9
Quetiapine: This drug is available as immediate- and extended-release tablets.10 It is rapidly absorbed and cleared with a 6- to 7-hour elimination half-life. The extended-release tablets give a peak concentration of 6 hours. For both forms, an active metabolite, norquetiapine, represents about half of the active drug in circulation and has a 12-hour elimination half-life. Therefore, a once-daily dose of 25 mg is recommended for the extended-release tablet and 25 mg bid for the immediate-release tablet. In certain cases, the dose has gone up to 800 mg.10
Quetiapine has strong histaminic, cholinergic, and alpha1-adrenergic binding, which are responsible for relatively high levels of sedation, dry mouth, and orthostatic hypotension.10 This drug also causes fewer EPS. Drug-drug interactions are not common with quetiapine, and no renal adjustment is necessary. As with other SGAs, weight gain is a side effect. QT prolongation is a problem, and this drug should not be combined with other medications that prolong QT intervals. In clinical trials for quetiapine, an increased risk of hypertension exists for pediatric patients. Baseline blood pressure and periodic monitoring are recommended in children and adolescents. Quetiapine may increase the risk of suicidal thoughts in children.10
Cost and Formulation
SGAs are more expensive than the FGAs, and prices vary based on the manufacturer, dispensing cost, and insurance coverage. Considering all dosage strengths, risperidone is the least expensive of all atypical antipsychotics. Unless there is a compelling reason not to use it, risperidone is considered first-line therapy. Several of the SGAs (risperidone, olanzapine, quetiapine, and ziprasidone) are available generically in the United States. In addition, several are available in orally disintegrating tablet (ODT), oral liquid, and IM formulations for administration in emergent situations. For example, an agitated, acutely psychotic patient can be treated with IM injection of a sterile solution. Rapid-acting forms can also be useful in patients who conceal pills in their mouth and later dispose of them. These tablets dissolve quickly in a minimal amount of saliva, making nonadherence difficult.5-10
Depot or long-acting, injectable drugs are used to treat patients unable to adhere to daily regimens. These drugs are injected in 2- to 4-week intervals.5-10
Conclusion
The reduced EPS side-effect profile of the SGAs has increased patients’ comfort levels and overall quality of life as compared with the FGAs. Both patients and society have benefitted by increased prescribing of the atypical antipsychotics for appropriate pediatric patients. Social functioning (e.g., school-work, employment, the ability to live independently) has been improved for patients as well. For these reasons, many psychiatrists now recommend atypical antipsychotics as first-line therapy for schizophrenia and other indications in pediatric patients.
REFERENCES
1. National Institute of Mental Health. Schizophrenia. How is schizophrenia treated? NIH Publication 09-3517. Revised 2009. www.nimh.nih.gov/health/publications/schizophrenia/index.shtml. Accessed April 13, 2015.
2. Newcomer JW. Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs. 2005;19:1-93.
3. Leucht S, Corves C, Arbter D, et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2008;373(9657):31-41.
4. Patel NC, Crismon ML, Hoagwood K, et al. Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry, 2005;44:548-556.
5. Pandina GJ, Aman MG, Findling RL. Risperidone in the management of disruptive behavior disorders. J Child Adolesc Psychopharmacol. 2006;16:379-392.
6. Risperdal (risperidone) prescribing information. Titusville, NJ: Janssen; December 2010. www.accessdata.fda.gov/drugsatfda_docs/label/2010/020272s063,020588s051,021346s040,021444s039lbl.pdf. Accessed February 12, 2015.
7. Abilify (aripiprazole) prescribing information. Rockville, MD: Otsuka America Pharmaceutical, Inc; February 2011. www.accessdata.fda.gov/drugsatfda_docs/label/2011/021436s029,021713s021,021729s014,021866s016lbl.pdf. Accessed February 12, 2015.
8. Zyprexa (olanzapine) prescribing information. Indianapolis, IN: Eli Lilly and Company; May 2010. www.accessdata.fda.gov/drugsatfda_docs/label/2010/020592s057,021086s036,021253s045lbl.pdf. Accessed February 20, 2015.
9. Invega (paliperidone) prescribing information. Titusville, NJ: Janssen; April 2011. www.accessdata.fda.gov/drugsatfda_docs/label/2011/021999s020s024lbl.pdf. Accessed February 14, 2015.
10. Seroquel (quetiapine) prescribing information. Wilmington, DE: AstraZeneca; December 2010. www.accessdata.fda.gov/drugsatfda_docs/label/2010/020639s052lbl.pdf. Accessed February 12, 2015.
To comment on this article, contact rdavidson@uspharmacist.com.






