US Pharm. 2009;34(8):HS-3-HS-8.
The incidence of testicular cancer is relatively low in the United States.1 This type of cancer accounts for roughly 2% of malignancies in the U.S. The American Cancer Society estimated that, in 2008, approximately 8,000 men would be diagnosed with testicular cancer and nearly 400 would die from it.2 The majority of new cases are diagnosed in males aged 15 to 35 years.3,4 Patients with localized disease have an overall survival rate approaching 100%, and patients with advanced disease have a rate of 70% to 80%.5,6
Germ cell tumors (GCTs) are the most common type of testicular neoplasm, with a 95% incidence.2,3 Carcinoma in situ and intratubular germ cell neoplasms advance to GCTs in roughly 5 years.7 Rarely occurring testicular neoplasms include sex cord-stromal tumors and lymphomas. GCTs may be found in extragonadal regions, most commonly the retroperitoneum and the mediastinum.8
Many risk factors have been proposed for the development of testicular cancer. The incidence is significantly higher in white men versus those of African or Asian descent.4,7 Known risk factors for the development of GCTs include cryptorchidism, cancer in the contralateral testis or history of testicular cancer, family history, and Klinefelter's syndrome.9
HISTOLOGY
The two types of GCTs are seminoma and nonseminoma. Seminomas are slow-growing neoplasms, whereas nonseminomas are more aggressive. Seminomas constitute approximately 40% of all GCTs and usually occur in the fourth decade of life.7 They may consist of syncytial trophoblastic cells that express human chorionic gonadotropin (HCG) on the cell surface. Thus, seminoma tumors may have an elevated HCG level at diagnosis and may be used as a serum tumor marker to evaluate disease progression after treatment.
Nonseminomas (accounting for the remaining 60% of GCTs) usually occur in the third decade of life.7 They consist of multiple cells and may include seminoma as one of their cell types. Other subtypes are yolk sac tumor, choriocarcinoma, embryonal carcinoma, and teratoma. Nonseminoma cell types produce high levels of tumor markers such as alpha-fetoprotein (AFP), HCG, and lactic dehydrogenase (LDH). Specifically, choriocarcinoma produces only HCG and yolk sac tumors produce only AFP; teratomas are derived from muscle, bone, and skin and produce no tumor markers. LDH relays tumor burden, growth rate, and cellular proliferation. All three tumor markers are useful for monitoring disease progression and treatment response, especially in postchemotherapy cycles. The respective half-lives of AFP and HCG are 5 days and 1 to 2 days. Slow declines in AFP and HCG levels are associated with poorer prognosis and disease progression.10
SIGNS, SYMPTOMS, AND DIAGNOSIS
Most testicular tumors are detectable upon physical examination. They present as a hard mass with diffuse testicular pain and swelling. Since infection is a common cause of testicular symptoms, epididymitis and orchitis must be ruled out. A course of antibiotics should be given; further evaluation is warranted if signs and symptoms persist despite antibacterial treatment. Regional metastasis can occur in the retroperitoneal lymph nodes; other sites of metastasis include the brain, liver, and bone. Advanced disease may cause abdominal, back, and bone pain, depending upon the location of the primary tumor and metastasis. Other symptoms include coughing, shortness of breath, and changes in mental status.3,4
If a testicular mass is suspected, a physical examination of the testes is performed. The contralateral testis can be used as a comparative tool. If differences in the testes are noted, testicular ultrasound should be conducted. The diagnostic workup should include a history, physical examination, chest x-ray, and chemistry profile with LDH and serum tumor markers. Additionally, computed tomography of the abdomen, pelvis, and chest should be performed. If signs and symptoms of advanced disease are present, MRI of the brain and bone should be added to the standard diagnostic workup to rule out metastasis. Histology of the primary tumor is determined by biopsy with radical orchiectomy.3,8
STAGING AND TREATMENT
Many organizations employ the tumor, nodes, and metastasis (TNM) classification system. In the U.S., the commonly used TNM staging system is the one developed by the American Joint Committee on Cancer. The TNM classification for testicular cancer differs from that for other solid tumors. It is unique in that it considers tumor markers in addition to the standard prognostic indicators.
According to the TNM system, stage I disease is limited to the testis and epididymis. Stage IB comprises tumor invasion into the spermatic cord or scrotum with or without vascular or lymphatic involvement. Stage II can be further subdivided into stages IIA, IIB, and IIC. Involvement of positive lymph nodes with a mass of up to 2 cm is stage IIA; in stage IIB, the lymph-node mass is more than 2 cm, but less than 5 cm; and stage IIC is used for a mass greater than 5 cm.2 Stage III involves distant metastasis, and a different categorization system--the International Germ Cell Cancer Collaborative Group (IGCCCG) classification--is used. The IGCCCG stratifies stage III patients according to three groups: good risk, intermediate risk, and poor risk (TABLE 1).11
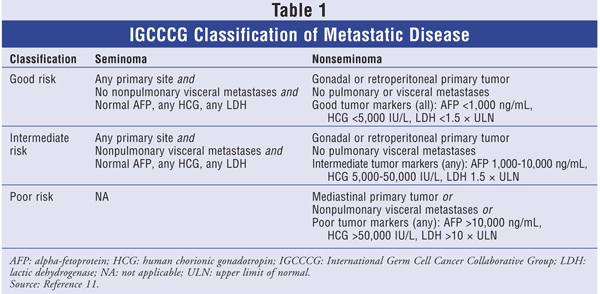
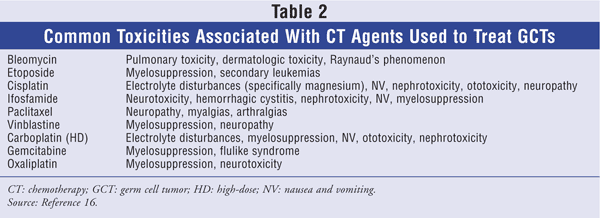
TABLE 2 lists common side effects associated with the various chemotherapy (CT) agents used to treat GCTs.
Stage I
Since stage I disease is limited to the testes, radical orchiectomy is the treatment of choice for both nonseminomas and seminomas. Management of disease may be considered following radical orchiectomy. Management options are determined by specific cell histology. Unlike most solid tumors, testicular GCTs have an excellent prognosis (approaching 100% survival), so the long-term effects of treatment must be considered when a plan is being developed. The patient should be included in the postorchiectomy treatment decision.5,7
Seminoma: The standard management method following orchiectomy is radiotherapy (RT). Because RT may expose patients to adverse events such as infertility, gastrointestinal (GI) problems, and secondary malignancies, other therapeutic options have been investigated. A trial was conducted to compare standard adjuvant RT with a single dose of carboplatin in stage I seminoma.12 The primary endpoint was relapse-free survival rate. Patients were randomly assigned to receive RT (n = 904) or one dose of carboplatin (n = 573), with the dose based on an AUC of 7 mg/mL/min. The two treatment groups had similar relapse-free survival rates at 2 and 3 years.
Although this has been the only trial to examine single-dose CT versus RT (additional studies are ongoing), the favorable result for CT gives patients another option besides RT. The adverse events associated with these treatments may figure significantly in a patient's decision. The single dose of carboplatin is administered at an AUC of 7 mg/mL/min (AUC = 7) and infused over 30 minutes. The most common side effects associated with carboplatin are myelosuppression (neutropenia and thrombocytopenia), electrolyte disturbances, and nausea and vomiting. These side effects are not of great concern since only a single dose is administered.
Nonseminoma: Because nonseminomas are highly aggressive, they are extremely sensitive to CT. The three postorchiectomy management options are surveillance, nerve-sparing retroperitoneal lymph node dissection (NS-RPLND), and adjuvant CT.5
Mortality rates exceed 90% in patients who receive radical orchiectomy alone. Surveillance is an option for patients who do not want to experience the side effects of CT or who undergo NS-RPLND. Surveillance has up to a 12% chance of relapse; however, CT at relapse can produce a 99% chance of survival. This method requires the patient to be extremely vigilant about follow-up, and the health care team must provide intensive monitoring. As this is a watch-and-wait treatment, the patient may experience some psychological distress regarding whether relapse will occur.5
In NS-RPLND, the lymph nodes are dissected with a nerve-sparing technique to prevent retrograde ejaculation and infertility in young men. This option eliminates recurrence in the retroperitoneal area, with overall relapse occurring in 3% to 4% of patients.3,5
Prior to the mid-1970s, the complete response rate (CRR; referring to the disappearance of all radiographic, biochemical, and clinical evidence of disease) for CT was roughly 25%. With the introduction of cisplatin in the mid-1970s, the CRR increased to 80%. A pivotal trial using a regimen comprising vinblastine, cisplatin, and bleomycin plus surgery produced a CRR of more than 80%. Major toxicities associated with this regimen were nephrotoxicity, neurotoxicity, and pulmonary adverse events.6 After this regimen was established, several studies investigated how to minimize adverse events without compromising efficacy. Trials substituting etoposide for vinblastine concluded that etoposide had equal efficacy and a substantially reduced incidence of neurotoxicity.6 Thus, bleomycin, etoposide, and cisplatin (BEP) became the standard first-line CT regimen for GCTs.6
With adjuvant therapy, only two cycles of BEP (BEP x 2) are necessary to produce a low (3%-4%) chance of relapse. Administration of more than two cycles exposes the patient to unwarranted adverse reactions and does not reduce the incidence of relapse in stage I nonseminomas.13,14 The BEP regimen is as follows: cisplatin 20 mg/m2 on days 1 to 5, etoposide 100 mg/m2 on days 1 to 5, and bleomycin 30 units on days 2, 9, and 16, with the cycle repeated every 21 days. The most common acute side effects are nephrotoxicity, ototoxicity, myelosuppression, and pulmonary toxicity. Patients should drink plenty of liquids to prevent long-term renal damage.
Cisplatin is considered a highly emetogenic CT agent. Patients must be premedicated with appropriate antiemetics. Bleomycin poses many potential lifetime toxicities. Pulmonary fibrosis, the most severe of all bleomycin toxicities, is due to cumulative lifetime doses in excess of 400 units. The diffusing capacity of the lung for carbon monoxide should be checked prior to administration of bleomycin; the frequency of routine monitoring is unclear. Patients experiencing respiratory problems upon physical examination should be further evaluated.15,16 Another long-term side effect of CT is irreversible ototoxicity (tinnitus, high-tone hearing loss). Etoposide may cause secondary malignancies; although the incidence is low (about 2%), this must be a consideration, particularly for young patients. With radical orchiectomy and CT, patients are at high risk for infertility and azoospermia, so young men who may wish to have children should consider banking their sperm.16
Stage II
Treatment of stage II disease is determined by specific histologic cell type.
Seminoma: Stages IIA and IIB should be treated with adjuvant RT postorchiectomy. If RT is contraindicated in a specific individual, then four cycles of etoposide and cisplatin (EP x 4) should be administered as adjuvant therapy. Stage IIC should be treated as advanced (stage III) disease, according to IGCCCG criteria. Treatment of stage IIC consists of either EP x 4 or three cycles of BEP (BEP x 3).2,17
Nonseminoma: Treatment of stages IIA and IIB varies according to tumor-marker levels and radiographic findings. Depending upon these factors, options are CT with either EP x 4 or BEP x 3; NS-RPLND; surveillance; or a combination. Stage IIC is treated as advanced disease, with initial platinum-based CT.2,17
Stage III
As noted earlier, patients with advanced disease are stratified into good-risk, intermediate-risk, or poor-risk status (TABLE 1). Risk type depends on the following variables: primary tumor, areas of metastasis, and serum tumor markers. Treatment options vary according to risk type. Five-year survival rates for good-, intermediate-, and poor-risk categories are 90%, 75%, and 40% to 50%, respectively.2,17
Seminoma: Because seminomas generally have a better prognosis than nonseminomas, only the good-risk and intermediate-risk categories apply to stage III seminomas (TABLE 1).11 Treatment is based on risk level and includes initial therapy with either EP x 4 or BEP x 3. If residual disease is present, additional RT or surgery is warranted.2,17
Nonseminoma: Platinum CT is highly efficacious against GCTs in patients with good risk. In these cases, cisplatin is the platinum of choice. In an effort to reduce adverse events such as nephrotoxicity, several studies have compared carboplatin versus cisplatin in this setting. In these studies, regimens including carboplatin resulted in increased relapses and deaths compared with cisplatin-containing regimens.18,19 Either BEP x 3 or EP x 4 is warranted in good-risk patients. As testicular GCTs are curable even in the metastatic setting, delayed toxicities are a major concern.
Several studies have investigated whether toxicities can be minimized by eliminating cycles or certain CT agents. A pivotal trial comparing EP x 4 with four cycles of BEP (BEP x 4) found no difference in overall survival; however, less pulmonary toxicity occurred in the EP group.20 This trial showed that EP x 4 can be administered without compromising treatment. If a patient has any risk factors for developing bleomycin-associated pulmonary toxicity, the EP regimen should be considered.20 Another trial examined the elimination of one cycle of BEP (BEP x 4 vs. BEP x 3) in good-risk patients. There was no difference in overall survival or disease-free progression, but major toxicities were reduced with BEP x 3. This led to the standard BEP x 3 regimen for good-risk patients.21 A study comparing EP x 4 with BEP x 3 concluded that there were no differences in efficacy, suggesting that EP x 4 is an alternative to BEP x 3 in good-risk patients with pulmonary-toxicity risk factors such as age over 40 years, cigarette smoking, poor renal function, and high oxygen exposure.22
Survival rates for intermediate-risk and poor-risk patients are significantly lower than for good-risk patients, so first-line CT in these settings consists of BEP x 4.2 High-dose CT (HDCT) plus stem-cell support has been compared with the standard BEP regimen as first-line treatment in intermediate-prognosis and poor-prognosis patients. A recent randomized trial assessed BEP x 4 versus BEP x 2 followed by two tandem HDCT regimens consisting of etoposide, carboplatin, and cyclophosphamide.23 CRRs were similar between the two groups, and there were no differences in overall and disease-free survival after a median of 51 months. As expected, adverse events were significantly greater in the HDC plus stem-cell support arm. These results indicate that BEP x 4 should remain the first-line standard of care for patients with intermediate and poor risk.23
SALVAGE THERAPY
As previously mentioned, testicular cancer is highly curable. Most patients achieve a complete response (CR) with initial platinum-based therapy. Relapse occurs in fewer than 10% of patients.16 Relapse can be detected by clinical assessment, tumor markers, and radiologic imaging. Patients who relapse within 4 weeks of the last course of a cisplatin-based regimen are considered refractory and have an extremely poor prognosis.
Common salvage regimens are summarized in TABLE 3. First-line regimens include ifosfamide or cisplatin plus either vinblastine or etoposide. As paclitaxel has shown some single-agent activity in refractory germ-cell neoplasms, paclitaxel combined with other CT agents has been studied. One trial evaluated the efficacy of four cycles of paclitaxel, ifosfamide, and cisplatin (TIP) as first-line salvage therapy in refractory GCTs.24 Forty-six patients with refractory GCTs were treated with TIP and prophylactic colony-stimulating growth factor (CSF). CR was achieved by 32 patients (70%), and 29 patients were disease-free at a median of 69 months. Progression-free survival rate at 2 years was 65%. Neutropenia was the primary toxicity despite prophylactic CSF. Severe neurotoxicity was seen in three patients; however, this may be attributed to previously administered cisplatin rather than paclitaxel therapy.24
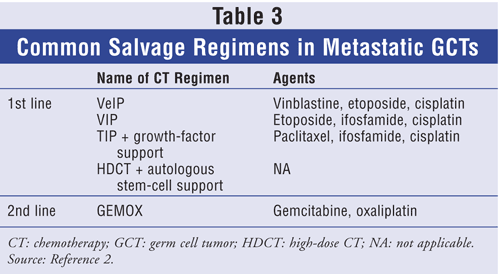
HDCT plus hematopoietic stem-cell rescue has been studied as first-line salvage treatment, with promising results. A retrospective study was conducted to evaluate patients with metastatic disease who had failed cisplatin-based treatment.25 The 184 patients received two consecutive doses of high-dose carboplatin and etoposide for 3 days, followed by autologous stem-cell rescue. Complete remission was achieved in 116 patients at a median follow-up of 48 months. Toxicities, which were minimal, included acute leukemia and GI problems.25
Patients who have failed two platinum-based regimens or autologous stem-cell transplantation have an extremely poor prognosis. Both gemcitabine and oxaliplatin have shown single-agent activity in testicular GCT. Two prospective studies have explored the combination of gemcitabine and oxaliplatin (GEMOX) in patients who were cisplatin-refractory or failed HDCT plus autologous stem-cell transplantation.26,27 In one study, 28 patients were assessed for response, and nine patients (32%) achieved an overall response.26 Another study examined 35 patients treated with GEMOX; results were similar to those in the previously mentioned trial, with an overall response rate of 43%. Toxicities involved mainly myelosuppression (mostly leukopenia and thrombocytopenia). Some patients also had neuropathy, but this was most likely due to previously administered cisplatin, as patients received only an average of two to three cycles of GEMOX.27
THE PHARMACIST'S ROLE
As testicular cancer is highly curable in both early and advanced stages, the prevention and management of acute and long-term toxicities are extremely important. Pharmacists play a valuable role in the management of these toxicities. There are numerous opportunities for intervention. Patients should be counseled as to the importance of maintaining adequate hydration when receiving platinum-based therapies to prevent renal toxicity. A detailed medication history should be obtained prior to therapy initiation to determine the necessity of discontinuing any drugs that may potentiate the development of nephrotoxicity and neurotoxicity. Patients with risk factors for developing pulmonary toxicity should be identified, and a regimen of EP should be considered. Appropriate antiemetics should be recommended for the prevention of nausea and vomiting. Electrolytes should be routinely monitored and replaced accordingly. Education by pharmacists is imperative in this setting to prevent toxicities without compromising high survival rates.
REFERENCES
1. Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242-254.
2. National Cancer Institute. Testicular cancer.
www.cancer.gov/cancertopics/
3. NCCN Clinical Practice Guidelines in Oncology. Testicular cancer. V2.2009.
www.nccn.org/professionals/
4. Bosl GJ, Bajorin DF, Sheinfeld J, et al. Cancer of the testis. In DeVita VT Jr, Hellman S, Rosenberg SA, eds. Cancer: Principles and Practice of Oncology. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001:1491-1513.
5. Jones RH, Vasey PA. Part I: Testicular cancer--management of early disease. Lancet Oncol. 2003;4:730-737.
6. Jones RH, Vasey PA. Part II: Testicular cancer--management of advanced disease. Lancet Oncol. 2003;4:738-747.
7. Krege S, Beyer J, Souchon R, et al. European Consensus Conference on Diagnosis and Treatment of Germ Cell Cancer: a report of the second meeting of the European Germ Cell Cancer Consensus Group (EGCCCG): Part I. Eur Urol. 2008;53:478-496.
8. Gori S, Porrozzi S, Roila F, et al. Germ cell tumours of the testis. Crit Rev Oncol Hematol. 2005;53:141-164.
9. Nichols CR, Heerema NA, Palmer C, et al. Klinefelter's syndrome associated with mediastinal germ cell neoplasms. J Clin Oncol. 1987;5:1290-1294.
10. Mazumdar M, Bajorin DF, Bacik J, et al. Predicting outcome to chemotherapy in patients with germ cell tumors: the value of the rate of decline of human chorionic gonadotrophin and alpha-fetoprotein during therapy. J Clin Oncol. 2001;19:2534-2541.
11. International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594-603.
12. Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366:293-300.
13. Cullen M, Stenning S, Parkinson MC, et al. Short-course adjuvant chemotherapy in high-risk stage I nonseminomatous germ cell tumors of the testis (NCGCTT): a Medical Research Council report. J Clin Oncol. 1996;14:1106-1113.
14. Böhlen D, Borner M, Sonntag RW, et al. Long-term results following adjuvant chemotherapy in patients with clinical stage I testicular nonseminomatous malignant germ cell tumors with high risk factors. J Urol. 1999;161:1148-1152.
15. Einhorn LH, Foster RS. Bleomycin, etoposide, and cisplatin for three cycles compared with etoposide and cisplatin for four cycles in good-risk germ cell tumors: is there a preferred regimen? J Clin Oncol. 2006;24:2597-2598.
16. Feldman DR, Bosl GJ, Sheinfeld J, Motzer RJ. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672-684.
17. Krege S, Beyer J, Souchon R, et al. European Consensus Conference on Diagnosis and Treatment of Germ Cell Cancer: a report of the second meeting of the European Germ Cell Cancer Consensus Group (EGCCCG): Part II. Eur Urol. 2008;53:497-513.
18. Bajorin DF, Sarosdy MF, Pfister DG, et al. Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol. 1993;11:596-606.
19. Horwich A, Sleijfer DT, Fossa SD, et al. Randomized trial of bleomycin, etoposide, and cisplatin compared with bleomycin, etoposide, and carboplatin in good-prognosis metastatic nonseminomatous germ cell cancer: a Multiinstitutional Medical Research Council/European Organization for Research and Treatment of Cancer Trial. J Clin Oncol. 1997;15:1844-1852.
20. de Wit R, Stoter G, Kaye SB, et al. Importance of bleomycin in combination chemotherapy for good-prognosis testicular nonseminoma: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group. J Clin Oncol. 1997;15:1837-1843.
21. de Wit R, Trevor Roberts J, Wilkinson PM, et al. Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol. 2001;19:1629-1640.
22. Culine S, Kerbrat P, Kramar A, et al. Refining the optimal chemotherapy regimen for good-risk metastatic nonseminomatous germ-cell tumors: a randomized trial of the Genito-Urinary Group of the French Federation of Cancers Centers (GETUG T93BP). Ann Oncol. 2007;18:917-924.
23. Motzer RJ, Nichols CJ, Margolin KA, et al. Phase III randomized trial of conventional-dose chemotherapy with or without high-dose chemotherapy and autologous hematopoietic stem-cell rescue as first-line treatment for patients with poor-prognosis metastatic germ cell tumors. J Clin Oncol. 2007;25:247-256.
24. Kondagunta GV, Bacik J, Donadio A, et al. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ cell tumors. J Clin Oncol. 2005;23:6549-6555.
25. Einhorn LH, Williams SD, Chamness A, et al. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. New Engl J Med. 2007;357:340-348.
26. Pectasides D, Pectasides M, Farmakis D, et al. Gemcitabine and oxaliplatin (GEMOX) in patients with cisplatin-refractory germ cell tumors: a phase II study. Ann Oncol. 2004;15:493-497.
27. Kollmannsberger C, Beyer J, Liersch R, et al. Combination chemotherapy with gemcit abine plus oxaliplatin in patients with intensively pretreated or refractory germ cell cancer: a study of the German Testicular Cancer Study Group. J Clin Oncol. 2004;22:108-114.





