US Pharm. 2007:32(7):HS-26-HS-36.
Heparin is one of the oldest drugs currently
in widespread clinical use. It is a heterogeneous mixture of branched
glycosaminoglycans, which was discovered to have antithrombotic properties
almost 100 years ago.1 Heparin was originally isolated from canine
liver cells, hence its name (hepar, or hpar, is Greek for liver
). It was found that the extract of canine liver was an inhibitor of
coagulation.2 It was then demonstrated by Brinkhous et al. that
heparin was an indirect anticoagulant, requiring a plasma cofactor.2
In 1968, this cofactor was named antithrombin III by Abildgaard and is
now referred to as AT.3 The main anticoagulation effect of
heparin is mediated by the heparin–AT interaction. The mechanism of action for
this interaction was elucidated in the 1970s by Rosenberg and Bauer and
Lindahl et al.4,5
This article reviews the
pharmacology, pharmacokinetic, and pharmacodynamic parameters of
unfractionated heparin (UFH); current clinical uses and common approaches to
UFH dosing; adverse effects and limitations of UFH; and current monitoring
practices, including the recent rise in use of the anti–factor Xa heparin
assay, a possible new standard of care in monitoring UFH.
Mechanism of Action and
Pharmacology
UFH is
heterogeneous in respect to molecular size, anticoagulant activity, and
pharmacokinetic properties. The molecular weights of these molecules range
from 5,000 to 30,000 Da, with a mean molecular weight of 15,000 Da (~45
monosaccharide chains).6 Only about one third of an administered
dose of UFH binds to AT, and this fraction is responsible for most of its
anticoagulant activity. UFH produces its major anticoagulant effect
by inactivating thrombin and activated factor X (factor Xa) through
an antithrombin-dependent mechanism. UFH binds to antithrombin
through a high-affinity pentasaccharide, leading to a
conformational change. The UFH-antithrombin complex is 100 to 1,000 times more
potent as an anticoagulant, compared with antithrombin alone.7
Antithrombin inhibits the activity of several clotting factors, including
factors IXa, Xa, and XIIa and thrombin. The UFH-antithrombin complex, through
its action of thrombin, not only prevents fibrin formation but also inhibits
thrombin-induced activation of factors V and VIII.8
In order for the UFH molecule
to inactivate thrombin, it must form a ternary complex between antithrombin
and thrombin. This is done by way of UFH binding to antithrombin, which causes
a conformational change that results in the exposure of its active site.
Molecules of heparin with fewer than 18 saccharides lack the chain
length to bridge between thrombin and antithrombin and, therefore,
are unable to inhibit thrombin. Only molecules that contain more than 18
saccharides are able to bind to antithrombin and thrombin simultaneously.9
After heparin has produced its
effect, it uncouples from antithrombin and quickly recouples with another
antithrombin molecule. The UFH–anti thrombin complex is unable to
inactivate thrombin or factor Xa within a formed clot or clots that are bound
to surfaces due to its relatively large size. Thus, UFH only prevents the
growth and propagation of a formed thrombus and allows the patient's own
thrombolytic system to degrade the clot.10
Commercially available UFH
preparations are derived from bovine lung or porcine intestinal mucosa.
Although some differences exist between these two preparations, no differences
in antithrombotic activity have been demonstrated. The Institute for Safe
Medication Practices includes heparin among its list of drugs that have a
heightened risk for causing significant patient harm when used in error.9
Pharmacokinetics
The biologic
activity and bioavailability of UFH is limited by its biologic propensity to
bind to plasma proteins, platelet factor-4, macrophages, fibrinogen,
lipoproteins, and endothelial cells.9 This may be a plausible
reason for substantial inter- and intrapatient variability observed in the
anticoagulation response to UFH. Rapid changes in the circulating levels of
the aforementioned heparin-binding proteins occur in patients who are acutely
ill or have active thrombosis. These patients will frequently appear to have
heparin resistance, requiring higher doses of UFH to achieve a therapeutic
response.9,11
Due to its large molecular size and
anionic structure, UFH is not absorbed reliably in the gastrointestinal tract
when taken orally. Intramuscular administration is discouraged, due to erratic
absorption, and may result in large hematomas. The bioavailability of
subcutaneous UFH is dose-dependent. The bioavailability ranges from 30% at
lower doses to as much as 70% at higher doses. After subcutaneous injection,
the anticoagulation effect is usually around one to two hours. When there is a
need for rapid anticoagulation, heparin may be given intravenously. Following
direct intravenous injection, the onset of anticoagulation activity is
immediate or occurs during the start of continuous intravenous infusion of
full doses of UFH. Subcutaneous administration is not recommended for rapid
anticoagulation due to its unpredictable absorption and delayed onset.9,12
The volume of distribution of UFH
(60 mL/kg) is similar to that of blood volume. UFH appears to be extensively
bound to low-density lipoprotein, globulins, and fibrinogen. UFH does not
cross placenta and is not distributed into breast milk. The dose required to
achieve a therapeutic anticoagulation response is correlated to weight.
Patients who are obese do not have a proportional increase in blood volume
relative to body weight. Nonetheless, when calculating initial heparin doses
for obese patients, it is unclear if the patients' actual or adjusted body
weight should be used.12
The plasma half-life of UFH is
approximately 30 to 90 minutes in healthy adults; however, the half-life is
dose dependent and increases with increasing doses. Several studies using
heparin sodium have demonstrated a shorter half-life in patients with a
pulmonary embolism, compared with healthy individuals and those with other
thrombotic disorders. In patients with liver impairment, the plasma half-life
is also decreased; however, it may be prolonged in cirrhotic patients. The
half-life of UFH may be slightly prolonged in anephric patients or patients
with severe renal impairment.11,12
The metabolism of UFH is not
fully understood, but the drug appears to be removed from the circulation
mainly by the reticuloendothelial system and may localize on arterial and
venous endothelium. There are two primary mechanisms for the elimination of
UFH. Depending on the dose and size of UFH, the elimination is related to
these two mechanisms. Low doses of UFH are cleared mostly by a saturable,
rapid, zero-order process. As part of this process, heparinases and
desulfatases enzymatically inactivate heparin molecules that are bound to
endothelial cells and macrophages, reducing them to smaller and less sulfated
molecules. UFH is also eliminated renally. This is a first-order process that
is slower and nonsaturable and predominantly occurs at very high doses.
Routine regimens of UFH comprise a combination of these two mechanisms for
elimination. Renal and hepatic dysfunction reduce the rate of clearance of UFH.
11,12
Clinical Uses and Dosing
UFH is used for
prophylaxis and treatment of venous thrombosis disorders. UFH may be used for
prophylaxis and treatment of pulmonary embolism; treatment of embolization
associated with atrial fibrillation and/or prosthetic heart valve replacement;
for prophylaxis and treatment of peripheral arterial embolism; for prophylaxis
of postoperative deep vein thrombosis and pulmonary embolism in patients
undergoing major abdominal or thoracic surgery who are at risk for
thromboembolism; and in the diagnosis and treatment of acute and chronic
consumptive coagulopathies.9 UFH may also be used to prevent
activation of the coagulation mechanism during arterial and cardiac surgery
and as blood passes through an extracorporeal circuit in dialysis procedures.
In addition, UFH is used in blood samples drowned for laboratory purposes and
as an in vitro anticoagulant in blood transfusions. Adjunctive anti
thrombotic therapy with UFH has also been used in patients with unstable
angina or non–ST-segment elevation/non–Q-wave myocardial infarction receiving
platelet glycoprotein-receptor inhibitors.11
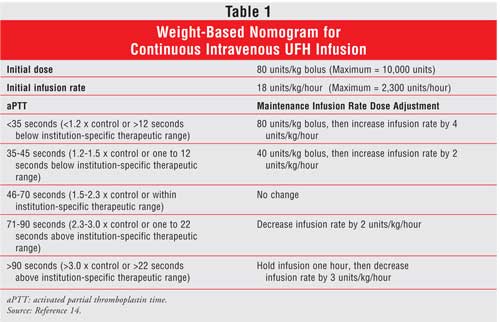
The dose and route of administration of UFH are based on the indication, the therapeutic goals, and the individual patient response to therapy.9 For the prevention of venous thromboembolism, UFH is given by subcutaneous injection in the abdominal fat layer. The typical dose for prophylaxis is 5,000 units every eight to 12 hours. A weight-based intravenous bolus dose followed by a continuous infusion is preferred when a patient requires immediate and full anticoagulation ( Table 1).13 A relationship has been reported between the dose of UFH administered and both its efficacy and safety.14,15 Thus, the dose of UFH must be adjusted by activated partial thromboplastin time (aPTT) or, when very high doses are given, by activated clotting time. Even though a weight-based approach to UFH dosing is superior, some clinicians still use the time-honored standard dosing regimens. Evidence from clinical trials demonstrates that the weight-based dosing protocols increase the proportion of patients who achieve a therapeutic response in the first 24 hours of therapy and lower the number of recurrent thrombotic events.13
Adverse Effects
Hemorrhage, the
major adverse effect of UFH therapy, is an extension of the pharmacologic
action of the drug and may range from minor local ecchymoses to major
hemorrhagic complications. Although there is a strong correlation between
subtherapeutic aPTT values and recurrent thromboembolism, the relationship
between supratherapeutic aPTT and bleeding is not as clear.16
Occurrence of bleeding complications is approximately 1.5% to 20% in patients
receiving UFH. The risk of bleeding is related to treatment intensity. Major
bleeding episodes occur more frequently with full-dose than with low-dose UFH
therapy and have been reported more frequently with intermittent intravenous
injection than with continuous intravenous infusion of the drug. The risk of
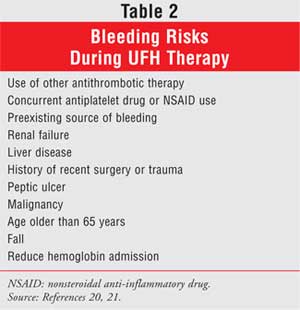
UFH-induced hemorrhage is increased by the presence of concomitant bleeding
risks (Table 2).
Another common side effect of UFH is
thrombocytopenia, which is defined as a platelet count of less than 150,000/mm
3. This side effect is often of no clinical significance and has been
reported to occur at an incidence of 30% or lower. Thrombocytopenia with UFH
therapy does not appear to be dose-related and has been reported to occur more
frequently with UFH prepared from bovine lung tissue than that prepared from
porcine intestinal mucosa. Two forms of acute, reversible thrombocytopenia
have been reported with UFH. Heparin-associated thrombocytopenia generally
occurs within the first few days of treatment in a heparin-naïve patient. This
condition is benign and mild, with platelet counts rarely dropping below
100,000/mm3. Mild thrombocytopenia may remain stable or reverse
even though UFH therapy is continued. The other form is heparin-induced
thrombocytopenia, which usually occurs five to 10 days after UFH treatment is
started. Heparin-induced thrombocytopenia is a serious drug-induced problem
requiring immediate intervention. This condition presents with a progressive
fall in platelet counts and, in some cases, thromboembolic complications. In
patients receiving UFH therapy, platelet counts should be monitored every one
to two days, and the patient should be evaluated for heparin-induced
thrombocytopenia if the platelet count drops by more than 50% or to below
100,000/mm3.20
Long-term UFH therapy has been
reported to cause delayed transient alopecia, priapism, and suppression of
aldosterone synthesis with subsequent hyperkalemia. Suppression of renal
function has also been reported following long-term, full-dose UFH therapy.
9 Osteoporosis and spontaneous fractures of the vertebral column have
been reported in patients receiving large daily doses (?10,000 units) of
UFH for longer than six months.21
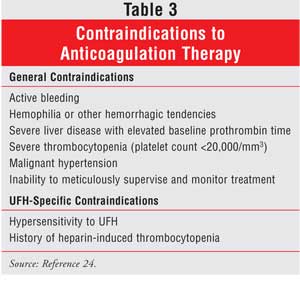
The occurrence of allergic reactions to heparin is rare. Hypersensitivity, which can be generalized, may be manifested by chills, fever, pruritis, urticaria, asthma, rhinitis, lacrimation, headache, nausea, vomiting, and anaphylactoid reactions, including shock. Contraindications to anticoagulant therapy, including UFH, are listed in Table 3.
Therapeutic Monitoring
Due to the
unpredictable anticoagulant response among patients given UFH, close
monitoring is required. Several tests are available to monitor UFH therapy
including whole blood clotting time, aPTT, activated clotting time,
anti–factor Xa activity, and plasma heparin concentrations. The aPTT is the
most widely used test to determine the degree of anticoagulation with UFH when
usual therapeutic doses are used. It is inexpensive, automated, and usually
available 24 hours a day. When very high doses of UFH are used in association
with percutaneous coronary interventions and cardiopulmonary bypass surgery,
the activated clotting time has been used to monitor therapy.23
In the 1970s, aPTT, reported as a
clotting time in seconds, was set in the range of 1.5 to 2.5 times the control
value. This range was shown to be associated with a reduced risk of recurrent
thromboembolism. Thereafter, a therapeutic aPTT range of 1.5 to 2.5 times the
control value gained wide clinical acceptance.23,24
However, in the past 25 years, the
reagents and instruments used to determine the aPTT have changed. Today, there
are more than 300 laboratory methods in use, as well as more than 30 reagent
instrument combinations used in the U.S. Thus, there is wide variation in
responsiveness to anticoagulants among different laboratories. This
variability is highlighted by the observation that at a plasma heparin
concentration of 0.3 units/mL (by factor Xa inhibition), mean aPTT results
range from 48 to 108 seconds depending on the laboratory method employed. At
therapeutic heparin levels (i.e., 0.3 to 0.7 anti–factor Xa units), modern
thromboplastin reagents produce an aPTT ratio that ranges from 1.6 to 2.7 to
3.7 to 6.2 times the control value. Therefore, it is clear that the standard
aPTT therapeutic range of 1.5 to 2.5 times the control value for all reagents
and methods of clot detection may lead to the administration of subtherapeutic
doses of UFH.25 Furthermore, there is no standard definition for
the term control value, which may be interpreted to be either the
patients' baseline prior to treatment or the average aPTT in healthy
volunteers.25,26
Despite these limitations, the aPTT
is still the most common method used for monitoring UFH therapy. In patients
with venous thromboembolism, the therapeutic level of UFH, as measured by the
aPTT, must be reached within the first 24 hours. The importance of achieving
this therapeutic range within 24 hours has been confirmed.27 Not
being able to reach the therapeutic aPTT level in patients with venous
thromboembolism who are treated with UFH has been associated with a
statistically significant and clinically important increase in the risk of
subsequent recurrent thromboembolism. In addition, the aPTT should
be measured six hours after a bolus dose of heparin and the continuous
intravenous dose should be adjusted according to the result. Low dose,
subcutaneous, prophylactic UFH is not routinely monitored, since low levels of
UFH do not affect the aPTT.24,25
Numerous UFH dose-adjustment
nomograms have been developed, but none can be applied to all aPTT
reagents. Therefore, the therapeutic range must be tailored
accordingly. Standardization may be achieved by calibration against plasma
heparin concentration using a therapeutic range of 0.3 to 0.7
units/mL based on an anti–factor Xa heparin assay or a
heparin level of 0.2 to 0.4 units/mL by protamine sulfate
titration. Problems with standardizing aPTT monitoring have been discussed in
a review by Raschke et al. This review examined the methodological quality of
UFH administration in clinical trials comparing UFH and low-molecular-weight
heparin for the treatment of venous thrombosis. Of the sixteen studies that
were included in the review, only three used a properly validated aPTT
therapeutic range in order to make UFH dose adjustments. Eleven studies used
aPTT ranges that were 1.5 times the control value, which is associated with
subtherapeutic UFH levels. Findings indicated that the true efficacy of UFH in
clinical trials of venous thromboembolism has likely been underestimated,
since most of the studies used invalidated aPTT therapeutic ranges and,
therefore, incorrect UFH dosing.28
Recognizing the substantial
variability in the aPTT, the College of American Pathologists has joined the
American College of Chest Physician in recommending against the generalized
use of a fixed aPTT therapeutic range, such as 1.5 to 2.5 times the control
value. Using a generalized aPTT range would guarantee systemic errors in UFH
administration in institutions with different thromboplastin reagents.
Instead, these two organizations recommend that the therapeutic aPTT range be
calibrated specifically for each reagent lot/coagulometer by determining the
aPTT values that correlate with therapeutic UFH levels.17,23
Anti–Factor Xa Heparin Assay in
the Monitoring of UFH
The choice of assay
used for monitoring UFH therapy is based on clinical preference and
institutional availability.9,23 Monitoring UFH therapy can also be
assessed by measuring heparin level. The two most common assay systems
available for measuring heparin levels are neutralization and functional
assays. The protamine titration assay, which is the neutralization form, is
labor intensive, inconvenient, and not available in most institutions.
Functional assays, such as the heparin anti–factor Xa assay, are becoming
automated and increasingly available to guide clinical practice. The
anti–factor Xa heparin assay is useful in the monitoring of both UFH and
low-molecular-weight heparin and is a more direct measure of UFH activity,
which previously had been reserved for use as a reference standard.23
While in North America the aPTT
remains the most commonly used test to monitor UFH therapy, in some European
countries the anti–factor Xa heparin assay is commonly used. However, an
increasing number of hospitals across the U.S., including some of the largest
hospital groups, are transitioning to the anti–factor Xa heparin assay.
26 The limitations of the aPTT test are well established, and the reason
for this transition is quite clear: to get patients into the therapeutic range
more quickly with fewer adjustments in dosage and repeat tests.9
Although there have been few studies
comparing the aPTT test to the anti–factor Xa heparin assay, those that have
been conducted have shown superiority with the anti–factor Xa heparin assay.
In a study by Levine et al., a group of patients requiring
unusually high doses of UFH to achieve a therapeutic aPTT were
monitored by measuring either factor Xa or aPTT. Significantly
lower amountsof UFH were required by the patient group that was monitored by
factor Xa heparin levels compared with the patients monitored by aPTT. These
patients were equally protected and experienced a lower rate
of major bleeding.29 In another study, only 48% of patients
monitored by the aPTT test reached the therapeutic range within 24 hours,
compared to 90% of patients monitored by the anti–factor Xa heparin assay.
30 Yet another study, in which a new heparin protocol was investigated
with 197 patients monitored by the anti–factor Xa heparin assay, showed that
62% of patients were in the therapeutic range at seven to nine hours, and 87%
were in the therapeutic range at 16 to 24 hours.31
In conclusion, numerous studies
dating back to as early as the late 1980s have demonstrated that aPTT does not
appear to be a useful surrogate for heparin levels. The limitations
of aPTT are well established and do not reliably correlate with
heparin blood concentrations or antithrombotic effects.32 Despite
this, the vast majority of hospitals continue to use the aPTT test, with
availability and cost being the most stated reasons why. Today, however, with
advanced diagnostics, the automated anti–factor Xa heparin assay can be
performed on a routine basis.33 According to findings within the
literature, current recommendations on the use of anti–factor Xa
heparin levels should be expanded, since UFH therapy monitored with heparin
levels may be more effective and safe.
References
1. McLean J. The
thromboplastic action of cephalin. Am J Physiol. 1916;41:250-257.
2. Brinkhous KM, Smith
HP, Warner ED, et al. The inhibition of blood clotting: an unidentified
substance which acts in conjunction with heparin to prevent the conversion of
prothrombin into thrombin. Am J Physiol. 1939;125:683-687.
3. Abildgaard U. Highly
purified antithrombin III with heparin cofactor activity prepared by disc
electrophoresis. Scand J Clin Lab Invest. 1968;21:89-91.
4. Rosenberg RD, Bauer
KA. The heparin antithrombin system: a natural anticoagulant mechanism. In:
Colman RW, Hirsch J, Marder VJ, et al, eds. Homeostasis and Thrombosis:
Basic Principals and Clinical Practice. 3rd ed. Philadelphia PA: JB
Lippincott; 1994:837-860.
5. Lindahl U, Backstrom
G, Hook M, et al. Structure of the antithrombin-binding site of heparin.
Proc Natl Acad Sci U S A. 1979;76:3198-3202.
6. Johnson EA, Mulloy
B. The molecular weight range of commercial heparin preparations. Carbohydr
Res. 1976;51:119-127.
7. Lam LH, Silbert JE,
Rosenberg JD. The separation of active and inactive forms of heparin.
Biochem Biophys Res Commun. 1976;89:570-577.
8. Casu B, Oreste P,
Torri G, et al. The structure of heparin oligosaccharide fragments with high
affinity anti-(factor Xa) activity containing the minimal antithrombin
III-binding sequence. Biochem J. 1981;97:599-609.
9. Hirsh J, Raschke R.
Heparin and low-molecular-weight heparin: The Seventh ACCP Conference on
Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3
Suppl):188S-203S.
10. Danielsson A, Raub
E, Lindahl U, Bjork I. Role of ternary complexes, in which heparin binds both
antithrombin and proteinase, in the acceleration of the reactions between
antithrombin and thrombin or factor Xa. J Biol Chem. 1986;261:15467.
11. Bick RL. Heparin
and low-molecular-weight heparins. In: Bick RL, ed. Disorders of Thrombosis
and Hemostasis: Clinical and Laboratory Practice. Philadelphia: Lippincott
Williams & Wilkins; 2002: 359-377.
12. Bara L, Billaud E,
Garamond G, et al. Comparative pharmacokinetics of low molecular weight
heparin (PK 10169) and unfractionated heparin after intravenous subcutaneous
administration. Thromb Res. 1985;39:631-636.
13. Raschke RA, Reilly
BM, Guidry JR, et al. The weight-based heparin dosing nomogram compared with a
"standard care" nomogram. Ann Intern Med. 1993;119:874-881.
14. Hull RD, Raskob GE,
Hirsh J et al. Continuous intravenous heparin compared with intermittent
subcutaneous heparin in the initial treatment of proximal-vein thrombosis.
N Engl J Med. 1986;315:1109-1114.
15. Platelet
glycoprotein IIb/IIIa receptor blockade and low-dose heparin during
percutaneous coronary revascularization. The EPILOG Investigators. N Engl J
Med. 1997;336:1689-1696.
16. Hull RD, Raskob GE,
Rosenbloom D, et al. Heparin for 5 days as compared with 10 days in the
initial treatment of proximal venous thrombosis. N Engl J Med.
1990;322:1260-1264.
17. Levine MN, Raskob
G, Beyth RJ, et al. Hemorrhagic complications of anticoagulant treatment: the
Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest
. 2004;126(3 Suppl):287S-310S.
18. Juergens CP,
Semsarian C, Keech AC, et al. Hemorrhagic complications of intravenous heparin
use. Am J Cardiol. 1997;80:150-154.
19. Hull RD, Raskob GE,
Rosenbloom D, et al. Optimal therapeutic level of heparin therapy in patients
with venous thrombosis. Arch Intern Med. 1992;152:1589-1595.
20. Warkentin TE,
Barkin RL. Newer strategies for the treatment of heparin-induced
thrombocytopenia. Pharmacotherpy. 1999;19:181-195.
21. Dahlman TC.
Osteoporotic fractures and the recurrence of thromboembolism during pregnancy
and the puerperium in 184 women undergoing thromboprophylaxis with heparin.
Am J Obstet Gynecol. 1993;168:1265-1270.
22. Ansell J, Hirsh J,
Poller L, et al. The pharmacology and management of the vitamin K antagonists:
the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.
Chest. 2004;126(3 Suppl):204S-233S.
23. Olson JD, Arkin CF,
Brandt JT, et al. College of American Pathologists Conference XXXI on
laboratory monitoring of anticoagulant therapy: laboratory monitoring of
unfractionated heparin therapy. Arch Pathol Lab Med. 1998;122:782-798.
24. Colvin BT,
Barrowcliffe TW. The British Society for Haematology Guidelines on the use and
monitoring of heparin 1992: second revision. BCSH Haemostasis and Thrombosis
Task Force. J Clin Pathol. 1993;46:97-103.
25. Baglin T,
Barrowcliffe,TW, Cohen A, Greaves M; British Committee for Standards in
Haematology. Guidelines on the use and monitoring of heparin. Br J Haematol.
2006;133:19-34.
26. Bussey HI. Problems with
monitoring heparin anticoagulation. Pharmacotherapy.1999;19:2-5.
27. Hull RD, Raskob GE,
Brant RF, et al. The importance of initial heparin treatment on long-term
clinical outcomes of antithrombotic therapy. The emerging theme of delayed
recurrence. Arch Intern Med. 1997;157:2317-2321.
28. Raschke R, Hirsh J,
Guidry JR. Suboptimal monitoring and dosing of unfractionated heparin in
comparative studies with low-molecular-weight heparin. Ann Intern Med.
2003;138:720-723.
29. Levine MN, Hirsh J,
Gent M, et al. A randomized trial comparing activated thromboplastin time with
heparin assay in patients with acute venous thromboembolism requiring large
daily doses of heparin. Arch Intern Med. 1994;154:49-56.
30. Markey-Moore S,
Douglas JB, Roh JM. Adjusting UFH using the antifactor Xa heparin assay.
Department of Pharmacy: The Moses Cone Memorial Hospital. Presented at: The
Southeastern Residency Preceptor's Conference; Athens, GA; 2000.
31. Rosborough TK,
Shepherd MF. Achieving target antifactor Xa activity with a heparin protocol
based on sex, age, height, and weight. Pharmacotherapy. 2004;24:713-719.
32. Baker BA, Adelman
MD, Smith PA, Osborn JC. Inability of the activated partial thromboplastin
time to predict heparin levels. Time to reassess guidelines for heparin
assays. Arch Intern Med. 1997;157:2475-2479.
33. Kitchen S, Theaker
J, Preston FE. Monitoring unfractionated heparin therapy: relationship between
eight anti-Xa assays and a protamine titration assay. Blood Coagul
Fibrinolysis. 2000;11:137-144.
To comment on this article,
contact editor@uspharmacist.com.






