US Pharm. 2007;32(5):HS-5-HS-19.
Millions of people in the
United States undergo surgery or are injured each year.1 Yet, for
people from all different backgrounds and in various stages of life, as well
as those with underlying medical conditions, the treatment of pain is less
than ideal.2-10 This issue reflects deeply seated issues pertaining
to all levels of the health care system and society. Furthermore, undertreated
pain has important clinical, economic, and human outcomes. Effects include
increased catabolic demand, decreased movement, cough suppression, and shallow
breathing; increased use of medical resources; and reduced health-related
quality of life (including diminished physical functioning).11-17
Evidence indicates that cellular and molecular changes seen in chronic pain
begin to appear with the initial injury, supporting the observation that
undertreated acute pain is a risk factor for chronic pain and that acute and
chronic pain exist on a continuum.18
The systematic undertreatment
of pain represents a public health crisis in this country. While all health
care professionals must have knowledge of the tools used to help treat pain,
pharmacists have a particularly significant role because they are highly
visible and accessible members of the health care team. The purpose of this
article is to review clinical issues related to the pharmacotherapy of acute
pain that community-based pharmacists are likely to encounter. Before the
pharmacotherapy of acute pain is discussed, it is important to ensure the use
of a common language to help avoid the impassioned and often mistaken use of
vocabulary that contributes to existing pain management obstacles.
Defining Pain
According to the
International Association for the Study of Pain (IASP), pain is an unpleasant
sensory and emotional experience associated with actual or potential tissue
damage.19 Pain may be described in terms of this damage. The
definition of pain is often subjective, being whatever the person says it is,
existing whenever the person says it does.20
From these definitions, it is
clear that pain is a complex, multidimensional, subjective experience, and
that the relationship between tissue damage and pain intensity is variable.
Additionally, the inability to communicate the presence of pain does not in
any way suggest that pain is absent. Because pain is subjective, health care
providers must rely on the person's report, even when reported pain and
behavior do not seem to match.
While pain is often described
using terms like acute and chronic, these and other distinctions
can be misleading. For example, acute pain is often described as a recent
onset that tends to diminish with time, while chronic pain tends to last
longer than is expected for the injury to heal.21 Acute pain can be
long-lasting; people often do not conform to expectations; and mixed types of
pain can be present at the same time. For example, patients with cancer may
experience pain that is acute, chronic, or some hybrid of these concepts.
Other examples include a person with chronic arthritis pain who undergoes
surgery, a person with cancer-related pain who also experiences episodes of
breakthrough pain, or a person with low back pain who is injured in a car
accident. In each setting, the affected patient will experience pain with
mixed features.
Pain is commonly referred to
in terms that reflect the underlying location or pathophysiology, such as
nociceptive or neuropathic.19,20 Nociceptive pain
results from pressure, temperature, or chemical stimuli. This type of pain is
also classified as originating from skin, bones, muscle, and connective tissue
(somatic) or internal organs (visceral).20 In general, somatic pain
tends to be specifically located, while visceral pain is more diffuse. In
contrast to nociceptive origins, nerve or nervous system damage may result in
neuropathic pain.19 This type of pain may be central, as with some
poststroke syndromes, or peripheral, such as diabetic neuropathy or
postherpetic neuralgia.
Dependence, Tolerance, and
Addiction
Much of the
confusion about pain management involves these concepts. Yet, rather than
allowing this confusion to interfere with the ability and willingness of
clinicians to provide effective pain management, pharmacists and their
colleagues on the health care team, patients, and families must be educated
about these phenomena.
If a patient taking a drug
develops a withdrawal syndrome when that substance is suddenly removed, the
individual is physically dependent on that substance.22 For opioid
analgesics, the withdrawal syndrome generally includes signs of central
arousal, such as insomnia, irritability, and agitation. Patients may also
experience autonomic symptoms, including diarrhea, rhinorrhea, and sweating,
as well as muscle spasms, gastrointestinal cramping, and other painful
phenomena.
It is critically important to
understand that dependence is an expected physiologic response to use of
certain drugs and neither a sufficient nor a necessary aspect of addiction.
22-24 Although we often think of dependence relative to use of opioid
analgesics, this concept also applies to any other drug (or pharmacologic
class) for which suddenly stopping use is discouraged. Typically, the best way
to avoid development of a withdrawal syndrome in a person thought to be
dependent on a drug is to slowly decrease the dose.
Similarly, tolerance refers to
the need for increased doses to produce a particular effect.22 For
a given drug, however, a person may become tolerant to some effects but not to
others. For example, with the opioid analgesics, tolerance to sedation and
respiratory effects typically develops quickly, while people generally develop
tolerance to the constipating effects of these drugs slowly, if at all. For
this reason, a preventive bowel regimen is considered a routine part of
therapy for individuals expected to be on opioid analgesics for an extended
period of time.
Addiction is probably one of
the most misunderstood phenomena associated with the use of opioid analgesics.
As defined by the American Pain Society, American Society of Addiction
Medicine, and American Academy of Pain Medicine, this primary, chronic,
neurobiological disease has genetic, psychosocial, and environmental
dimensions.22-24 Addicted individuals may have impaired control
over their drug use, compulsive use of the substance, continued use despite
harm, and craving for the substance. Furthermore, evidence in biomedical
literature overwhelmingly indicates that the rate of iatrogenic addiction
among persons who are being treated for acute pain, and who do not have a
history of substance abuse, is vanishingly low.25-29 This evidence
and our understanding of addiction support the contention that people who use
opioid analgesics to relieve their pain on a mutually agreed-upon schedule
without aberrant behaviors, whose functioning and pain control are relatively
stable, and who are willing to consider various treatment options are unlikely
to become addicted.22-24 As a result, concern about causing a
patient to become addicted should not contribute to clinical decisions about
how to treat pain, nor to patients' willingness to use appropriately
prescribed analgesics.23,25-29
While evidence indicates that
the risk of iatrogenic addiction in persons who are treated for acute pain is
nearly zero, systematic undertreatment of pain--including use of subpotent
analgesics, dosing regimens that do not reflect the pharmacokinetics and
pharmacodynamics of the analgesic, and inappropriate reliance on as-needed use
of these drugs--is common. Moreover, not only does the systematic
undertreatment of pain unfairly penalize persons with pain, it can also
directly result in a phenomenon known as pseudoaddiction.30 In this
syndrome, the patient may (unsurprisingly) request analgesics before the next
scheduled dose, doctor-shop, and act in other ways that are seen in persons
who abuse substances. The distinction is that when an undertreated
individual's pain is appropriately treated, these aberrant behaviors
disappear. Rather than waiting for a problem to develop, however,
pseudoaddiction can be avoided by building trust between the patient and the
health care team, using analgesics on a regular schedule instead of an
as-needed basis, and using adjuvants and nondrug treatments.
Evidence of Undertreated
Pain
During the past
four decades, there have been numerous published reports of suboptimally
treated pain among persons with acute pain.2-10 Progress in
improving the care for these individuals has continued, but it has been done
slowly and fitfully and has been less successful than might be expected, given
the availability of potent analgesics and clinical practice guidelines to help
clinicians.3,31-33 Well-documented barriers to effective
evidence-based pain management include deficiencies in pre- and postgraduate
health professions education; incorrectly held attitudes and beliefs about
opioid analgesics, adverse effects, and pain itself; and fear of prosecution.
3,34-41
Pain Pharmacotherapy
Nonopioids:
These drugs include salicylates, acetaminophen, and the NSAIDs. They
are at least generally familiar to almost everyone, since they are nearly
ubiquitous in prescription and OTC medications. These drugs are used primarily
for mild to moderate pain, although in combination with opioids, they are
often used for more intense pain.
Acetaminophen is a centrally
acting analgesic that does not have significant anti-inflammatory activity,
nor does it affect platelets or gastric mucosa.42 Despite a
generally favorable toxicity profile, acetaminophen must be used cautiously
because it is potentially hepatotoxic, particularly in persons with hepatic or
renal disease, chronic alcoholism, or malnutrition. Even in otherwise healthy
adults, the maximum daily dose of acetaminophen from all sources should not
exceed 4,000 mg.42,43 This is important because acetaminophen is
used in fixed-combination drugs with opioid analgesics. While there is no set
ceiling dose for opioids, there is a clearly identified limit for
acetaminophen, which can result in an unnecessary, artificial barrier to
optimal analgesia.
Additionally, the rectal
absorption of acetaminophen is variable, and this can affect the doses needed
to provide pain relief. For example, although the recommended pediatric oral
dose of acetaminophen is 10 to 15 mg/kg every six hours, a rectal loading dose
of 40 mg/kg with maintenance doses of 20 mg/kg every six hours has been found
to be safe and effective in at least one study.44,45
NSAIDs are also commonly used
for a wide variety of painful conditions and have proven effective in treating
postoperative pain. As their name suggests, these agents inhibit central and
peripheral prostaglandin synthesis, diminishing inflammation. Yet, because
NSAIDs do not affect circulating pros taglandins, pain relief occurs
sooner than anti-inflammatory effects.18 As with acetaminophen, the
NSAIDs have a ceiling effect, beyond which therapeutic benefit does not
increase, but the risk of adverse effects, including nausea, vomiting, and
gastrointestinal bleeding, does increase. This observation is particularly
important, since NSAID use results in significant morbidity and mortality in
the U.S. Despite these well-described risks, the risk-benefit ratio of NSAIDs
remains generally favorable in terms of their therapeutic potential.
Cyclooxygenase-2 (COX-2)
Inhibitors: Many
questions remain about the possible role of COX-2 selective inhibitors in
clinical practice. While these drugs are similarly efficacious to nonselective
NSAIDS, the main argument for use of COX-2 inhibitors has always been safety,
and it is here that many unresolved issues persist. For example, rofecoxib and
valdecoxib were withdrawn from the U.S. market for safety concerns. In
addition, in a large, randomized trial, individuals with rheumatoid arthritis
or osteoarthritis who took celecoxib had fewer symptomatic upper GI ulcers and
related complications than individuals who took ibuprofen or diclofenac over
the first six months of use, although this benefit disappeared by the end of
one year of use.46,47 There is also some evidence suggesting that a
clinically important drug interaction may occur between warfarin and COX-2
inhibitors.48,49 Other compounds in this class are in various
stages of clinical development; thus, it remains to be seen whether the
benefit in persons with arthritis occurs immediately and if this effect is
broadly generalizable.
Opioids:
Without a doubt, opioids have an important and useful role in the treatment of
moderate to severe pain. Notably, these drugs should not be referred to as
narcotics, a term that has been associated with barriers to optimal pain
management and that fails to clearly identify the specific type of drug.23
Opioids are often classified
by their activity at mu, kappa, or delta receptors in the central nervous
system.50,51 Effects of the mu- and kappa-receptor agonists include
analgesia. Mu-agonists also affect mood and reward behavior, and while
kappa-active drugs may produce less respiratory depression and miosis, these
drugs are also associated with dysphoria. It is important to remember that in
the dosage range typically used to treat acute pain, the mu-receptor agonists
have no therapeutic dosage ceiling.50,51 Provided that the person
is getting pain relief and is not having intolerable side effects, the dose of
the opioid analgesic can be increased. Opioid analgesics also lack the adverse
effects associated with NSAIDs, and people who do not respond to one opioid
may still respond to another.
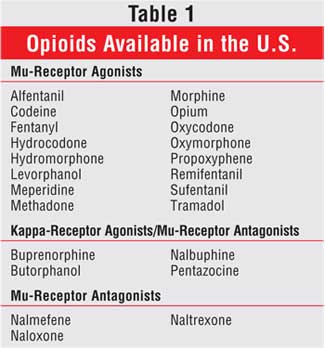
Currently available opioid
analgesics and antagonists are listed in Table 1. Most of the opioid
analgesics are mu-receptor agonists, although several are mu-receptor
antagonists and kappa-receptor agonists. The mixed-activity drugs (once
commonly called mixed agonist-antagonists) were designed to provide a
lower risk of respiratory depression and abuse but when used in equianalgesic
doses, their rate of adverse effects is comparable to that of the mu-receptor
agonists.50,51 Furthermore, there is a consistent dose-response
relationship with the mu-receptor agonists, but the kappa-receptor
agonist/mu-receptor antagonist drugs are not thought to possess that quality.
Opioids to Avoid:
The role for codeine, meperidine, and propoxyphene in acute pain management
is limited, regardless of the route of administration. Codeine is a prodrug
and must be converted to morphine via the cytochrome P-4502D6 pathway.53
About 10% of a codeine dose is converted to morphine, which is about 30%
bioavailable. As a result, 30 mg of codeine provides just 1 mg of morphine.
Persons who lack the ability to metabolize codeine to morphine get no
analgesia from the drug, although they are still at risk for dose-limiting
adverse effects, which commonly occur.51
Meperidine is about 1/10 as
potent as morphine on a milligram-to-milligram basis; thus, a 75-mg dose of
meperidine is equivalent to about 5 to 7.5 mg of morphine.3 A
dosing interval of four to six hours is often used for meperidine, but the
drug provides analgesia for 2.5 to three hours. As a result, 100 to 150 mg of
meperidine every three hours would be needed to provide analgesia equivalent
to 10 mg of morphine every four hours.3
Meperidine's active metabolite
normeperidine is renally eliminated. Normeperidine is neurotoxic and can cause
a variety of serious adverse effects, including seizures, even in persons with
normal renal function.23,53,54 Additionally, concomitant therapy
with meperidine and monoamine oxidase inhibitors (MAOI) (or use within two
weeks of discontinuation of the MAOI), including seligilene, is absolutely
contraindicated due to a risk of hypertensive crisis, hyperpyrexia, and
cardiovascular system collapse.55 Use of meperidine should be
avoided whenever possible. If use of this analgesic is unavoidable, American
Pain Society guidelines recommend use for no more than 48 hours and at doses
no more than 600 mg per 24 hours in persons with normal renal function.3
Propoxyphene has no clinical
advantages over acetaminophen.3,56 Like meperidine, propoxyphene
also has an active, toxic metabolite norpropoxyphene that accumulates in
persons with decreased renal function.53,57 This metabolite is also
associated with an increased incidence of falls in elderly individuals.58
Equianalgesic Conversion:
Morphine is the prototypical opioid analgesic. However, there are times when
it is desirable to use one of the other drugs in this class. For example, an
individual may be allergic or hypersensitive, intolerable adverse effects may
occur, or the drug may not provide the desired degree of pain relief.
Pharmacokinetic considerations may also have an impact. For example, neither
hydromorphone nor oxycodone have clinically active metabolites, so these
agents are often preferred in people with diminished renal function.
At equianalgesic doses, the
opioid analgesics have similar efficacy, although adverse-effect profiles may
vary. There are a variety of dose-conversion tables and methods available, and
different results are common depending on the method used. Some evidence also
suggests that conversion factors differ based on the drug used, the drug that
it is being converted to, and whether the person is opioid-naïve or
opioid-tolerant.21,59,60
A good example of this
phenomenon is methadone. While methadone was once used mainly for opioid
maintenance programs, its use as an analgesic has increased substantially over
the past few years. As a result, pharmacists in community practice are much
more likely to encounter its use. Methadone is generally considered to be
equipotent to morphine in opioid-naïve individuals, but its elimination
half-life is much longer than its biologic half-life, and it is also an N
-methyl-d-aspartate (NMDA) receptor antagonist. As a result, large decreases
in methadone doses (~90%) may be needed over the first few days after changing
analgesics. Failing to account for this phenomenon can contribute to serious
and even fatal adverse events.
Several methods for
calculating equianalgesic doses are used; some use tables in pharmacy
references commonly available, while others take relative potency and
pharmacokinetic parameters into account.21,59,60 Two of these
methods are shown in Table 2; however, it is important to recognize
that major differences between methods can result. For example, converting
from morphine to oxycodone using method 1 in the table indicates that 48 mg of
intravenous morphine is equivalent to 96 mg of oral oxycodone, while using
method 2 provides a result of 60 mg of oral oxycodone. One difference between
these approaches is that in the first method, 20 mg of oral oxycodone is
considered to be equivalent to 30 mg of oral morphine. This conversion factor
is frequently used, but for this to be true, oxycodone would have to be about
50% bioavailable, rather than 80%.21 Method 2, in contrast,
accounts for potency and bioavailability of these drugs.
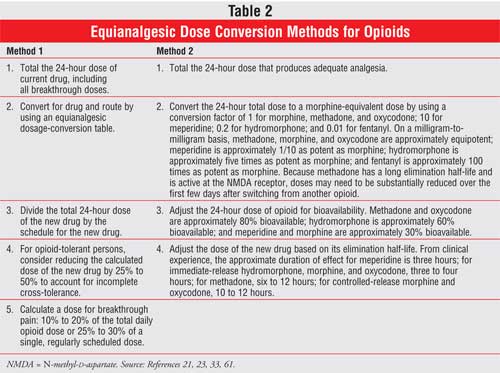
To help avoid cases of serious
overdose, one approach is to decrease the dose of the new opioid by
approximately 25% to 50% to account for incomplete cross-tolerance. However,
the percentage decrease depends on how well the person's pain is being
controlled, among other factors.61
Role of Community
Pharmacists
There is ample
evidence documenting pharmacists' impact in helping reduce adverse drug events
and their associated costs in hospitalized persons.61-63 Thus, it
is logical to question how to best assess the contribution that
community-based pharmacists can make to patient outcomes. An example of this
effort was described in a study on the feasibility of health outcomes
assessment in community pharmacy practices.64 Individuals who
participated in this project had been diagnosed with osteoarthritis,
rheumatoid arthritis, or low back pain, among other musculoskeletal disorders.
Study participants met with a pharmacist every three months for a year and
completed a survey that included condition-specific items and the SF-36, as
well as questions about the use of medical resources. The SF-36 is used to
estimate the effects of illness or medical conditions on health-related
dimensions of quality of life that are believed to be universally important
and are not age, treatment, or disease specific.65 Data collected
in this study help indicate how these conditions affect health-related quality
of life. There is no way to understand the effects of health care on
individuals unless health care providers ask. This information can help
identify people who experience adverse effects from their medications or who
need additional attention in order to improve their therapeutic regimen.
Possibly more important, these data show that it is possible to collect this
information in community pharmacies. Given the numerous demands on
pharmacists' time and attention, it is encouraging to see the potential of
community pharmacists to contribute to patient care.
There are a wide variety of
ways that pharmacists can help care for persons with pain. Their roles include
compounding and dispensing; serving as a resource for clinicians, patients,
and family members; advocating on behalf of patients suffering from pain; and
helping ensure continuity of care.66 As the number of people who
undergo outpatient surgical procedures rises and pressure increases to
discharge hospitalized individuals as soon as they are medically stable, this
latter point will become increasingly important.
Conclusion
The promise of
relief from pain exerts a powerful attraction, leading people in distress to
seek medical care. Pain is a universal part of being human, and yet, evidence
demonstrates that people from all backgrounds, stages of life, and levels of
health experience less than optimal treatment of their pain. This situation
exists for many reasons that pertain to our health care system and society.
Improving pain management is
complex and multidimensional--much like pain itself--and there are many
important challenges clinicians must face. Yet, there is good news: Because of
pharmacists' visibility and ready accessibility, there are ample opportunities
for us to become leaders in this effort.
References
1. Inpatient surgery. National Center for Health Statistics. Available at:
www.cdc.gov/nchs/fastats/insurg.htm. Accessed March 7, 2007.
2. Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in
outpatients with metastatic cancer. New Engl J Med. 1994;330:592-596.
3. Carr DB, Jacox AK, Chapman CR, et al. Clinical practice guideline number 1:
acute pain management: operative or medical procedures and trauma. Rockville,
MD: Agency for Health Care Policy and Research, 1992; AHCPR publication no.
92-0032.
4. Marks RM, Sachar EJ. Undertreatment of medical inpatients with narcotic
analgesics. Ann Intern Med. 1973;78:173-181.
5. Donovan M, Dillon P, McGuire L. Incidence and characteristics of pain in a
sample of medical-surgical inpatients. Pain. 1987;30:69-78.
6. Sriwatanakul K, Weis OF, Alloza JL, et al. Analysis of narcotic analgesic
usage in the treatment of postoperative pain. JAMA. 1983;250:926-929.
7. Warfield CA, Kahn CH. Acute pain management: programs in US hospitals and
experiences and attitudes among US adults. Anesthesiology.
1995;83:1090-1094.
8. Miaskowski C, Nichols R, Brody R, et al. Assessment of patient satisfaction
utilizing the American Pain Society's quality assurance standards on acute and
cancer-related pain. J Pain Symptom Manage. 1994;9:5-11.
9. SUPPORT Principal Investigators. A controlled trial to improve care for
seriously ill hospitalized patients. The Study to Understand Prognoses and
Preferences for Outcomes and Risks of Treatments (SUPPORT). JAMA.
1995;274:1591-1598.
10. Wolfe J, Grier HE, Klar N, et al. Symptoms and suffering at the end of
life in children with cancer. New Engl J Med. 2000;342:326-333.
11. Gottschalk A, Smith DS, Jobes DR, et al. Preemptive epidural analgesia and
recovery from radical prostatectomy. A randomized controlled trial. JAMA
. 1998;279:1076-1082.
12. Kiecolt-Glaser JK, Page GG, Marucha PT, et al. Psychological influences on
surgical recovery: perspectives from psychoneuroimmunology. Am Psychol.
1998;53:1209-1218.
13. Coley KC, Williams BA, DaPos SV, et al. Retrospective evaluation of
unanticipated admissions and readmissions after same day surgery and
associated costs. J Clin Anesth. 2002;14:349-353.
14. Bonica JJ. Importance of effective pain control. Acta Anaesthesiol Scand
. 1987;31(suppl 85):1-16.
15. Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome.
Brit J Anaesth. 2001;87:62-72.
16. Perkins FM, Kehlet H. Chronic pain as an outcome of surgery--a review of
predictive factors. Anesthesiology. 2000;93:1123-1133.
17. Morris DB. The Culture of Pain. Berkeley, CA: University of
California Press; 1993.
18. Carr DB, Goudas L. Acute pain. Lancet. 1999;353:2051-2058.
19. International Association for the Study of Pain. IASP pain terminology.
Available at: www.iasp-pain.org. Accessed March 11, 2007.
20. Pasero C, Paice JA, McCaffery M. Basic mechanisms underlying the causes
and effects of pain. In: McCaffery M, Pasero C, editors. Pain: Clinical
Manual. 2nd ed. St. Louis: Mosby; 1999.
21. Ready LB, Edwards WT, editors. Management of Acute Pain: A Practical
Guide. Seattle: IASP; 1992.
22. Savage SR, Joranson DE, Covington EC, et al. Definitions related to the
medical use of opioids: evolution towards universal agreement. J Pain
Symptom Manage. 2003;26:655-667.
23. McCaffery M, Pasero C, editors. Pain: Clinical Manual. 2nd ed. St.
Louis: Mosby; 1999:161-299.
24. McCaffery M, Pasero C, editors. Pain: Clinical Manual. 2nd ed. St.
Louis: Mosby; 1999:162, 429.
25. Porter J, Jick H. Addiction rare in patients treated with narcotics.
New Engl J Med. 1980;302:123. Letter.
26. Perry S, Heidrich G. Management of pain during debridement: a survey of US
burn units. Pain. 1982;13:267-280.
27. Brozovic M, Davies SC, Yardumian A, et al. Pain relief in sickle cell
crisis. Lancet. 1986;2:624-625.
28. Vichinsky EP, Johnson R, Lubin BH. Multidisciplinary approach to pain
management in sickle cell disease. Am J Ped Hematol Oncol.
1982;4:328-333.
29. Pegelow CH. Survey of pain management therapy provided for children with
sickle cell disease. Clinical Pediatrics. 1992;31:211-214.
30. Weissman DE, Haddox JD. Opioid pseudoaddiction--an iatrogenic syndrome.
Pain. 1989;36:363-366.
31. Benjamin LJ, Dampier CD, Jacox AK, et al. Guideline for the management of
acute and chronic pain in sickle-cell disease. APS clinical practice
guidelines series, no. 1. Glenview, IL: American Pain Society; 1999.
32. Simon L, Lipman A, Jacox A et al. Guideline for the Management of Pain
in Osteoarthritis, Rheumatoid Arthritis, and Juvenile Chronic Arthritis.
2nd ed. APS clinical practice guidelines series, no. 2. Glenview, IL: American
Pain Society; 2002.
33. Ashburn MA, Lipman AG, Carr D et al. Principles of Analgesic Use in the
Treatment of Acute Pain and Chronic Pain. 5th ed. Glenview, IL: American
Pain Society; 2003.
34. Jacox A, Carr DB, et al. Management of cancer pain: clinical practice
guideline, no. 9. Rockville, MD: Agency for Health Care Policy and Research,
1994; AHCPR publication no. 94-0592.
35. Lasch KE, Greenhill A, Wilkes G, et al. Why study pain? J Palliat Med.
2002;5:57-72.
36. Singh RM, Wyant SL. Pain management content in curricula of U.S. schools
of pharmacy. J Am Pharm Assoc. 2003;43:34-40.
37. McNicol E. Pharmacy and pain management: much work left to do. J Am
Pharm Assoc. 2003;43:343-344.
38. Furstenberg CT, Ahles TA, et al. Knowledge and attitudes of healthcare
providers toward cancer pain management: a comparison of physicians, nurses
and pharmacists in New Hampshire. J Pain Symptom Manage.
1998;15:335-349.
39. Bressler LR, Geraci MC, Schatz BS. Misperceptions and inadequate pain
management in cancer patients. DICP. 1991;25:1225-1230.
40. Ward SE, Goldberg N, Miller-McCauley V, et al. Patient-related barriers to
management of cancer pain. Pain. 1993;52:319-324.
41. Joranson DE, Berger JW. Regulatory issues in pain management. J Am
Pharm Assoc. 2000;40(5, suppl 1):S60-S61.
42. Drug Facts and Comparisons. Acetaminophen. Available at:
www.efactsweb.com. Accessed March 11, 2007.
43. Draganov P, Durrence H, et al. Alcohol-acetaminophen syndrome.
Postgraduate Med. 2000;107:189-195.
44. Buck ML. Perioperative use of high-dose rectal acetaminophen. Pediatric
Pharmacother. 2001;7:1-3.
45. Birmingham PK, Tobin MJ, Henthorn TK, et al. Twenty-four-hour
pharmacokinetics of rectal acetaminophen in children: an old drug with new
recommendations. Anesthesiology. 1997;87:244-252.
46. Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity
with celecoxib vs. nonsteroidal anti-inflammatory drugs for osteoarthritis and
rheumatoid arthritis: the CLASS study. A randomized controlled trial.
Celecoxib Longterm Arthritis Safety Study. JAMA. 2000;284:1247-1255.
47. Hrachovec JB, Mora M. Reporting of 6-month vs. 12-month data in a clinical
trial of celecoxib. JAMA. 2001; 286:2398. Letter.
48. Celebrex package insert. Available at: www.celebrex.com. Accessed March
11, 2007.
49. Schaefer MG, Plowman BK, Morreale AP, et al. Interaction of rofecoxib and
celecoxib with warfarin. Am J Health Syst Pharm.2003;60:1319-1323.
50. Twycross RG. Opioids. In: Wall PD, Melzack R, eds. Textbook of Pain
. 4th ed. New York: Churchill Livingstone; 1999: 1187-214.
51. Gutstein HB, Akil H. Opioid analgesics. In: Hardman JG, Limbird LE, Gilman
AG, editors. Goodman and Gilman's The Pharmacological Basis of Therapeutics
. 10th ed. New York: McGraw-Hill; 2001:569-619.
52. Michalets EL. Update: clinically significant cytochrome P-450 drug
interactions. Pharmacotherapy. 1998;18:84-112.
53. Latta KS, Ginsberg B, Barkin RL. Meperidine: a critical review. Am J
Ther. 2002;9:53-68.
54. Kaiko RF, Foley KM, Grabinski PY, et al. Central nervous system excitatory
effects of meperidine in cancer patients. Ann Neurol. 1983;13:180-185.
55. Quinn TE. Pain topics. Meperidine--what's all the fuss? Available at:
www.massgeneral.org/painrelief/Newsletter/prcvol2_2.pdf. Accessed March 11,
2007.
56. Oxford League table of analgesics in acute pain. Available at:
www.jr2.ox.ac.uk/bandolier/booth/painpag/Acutrev/Analgesics/Leagtab.html.
Accessed March 11, 2007.
57. Inturrisi CE. Clinical pharmacology of opioids for pain. Clin J Pain
. 2002;18:S3-S13.
58. Kamal-Bahal SJ, Doshi JA, Stuart BC, et al. Propoxyphene use by community
dwelling and institutionalized elderly Medicare beneficiaries. J Am Geriatr
Soc. 2003;51:1099-1104.
59. Souter KJ, Fitzgibbon D. Equianalgesic dose guidelines for long-term
opioid use: theroretical and practical considerations. Seminars in
Anesthesia, Perioperative Medicine and Pain. 2004;23:271-280.
60. Gammaitoni AR, Fine P, et al. Clinical application of opioid equianalgesic
data. Clin J Pain. 2003;19:286-297.
61. Montazeri M, Cook DJ. Impact of a clinical pharmacist in a
multidisciplinary intensive care unit. Critical Care Med.
1994;22:1044-1048.
62. Kucukarslan SN, Peters M, Mlynarek M, et al. Pharmacists on rounding teams
reduce preventable adverse drug events in hospital general medicine units.
Arch Intern Med. 2003;163:2014-2018.
63. Leape LL, Cullen DJ, Clapp MD, et al. Pharmacist participation on
physician rounds and adverse drug events in the intensive care unit. JAMA.
1999;282:267-270.
64. Osterhaus JT, Dedhiya SD, Ernst ME, et al. Health outcomes assessment in
community pharmacy practices: a feasibility project. Arthritis Rheum.
2002;47:124-131.
65. Ware Jr JE, Snow KK, Kosinski M, Gandek B. SF-36 health survey manual
and interpretation guide. Boston, MA: The Medical Outcomes Trust; 1993.
66. Strassels SA, McNicol E, Suleman R. Postoperative pain management: a
practical review, part 2. Am J Health Syst Pharm.2005;62:2019-2025.
To comment on this article, contact editor@uspharmacist.com.





