US Pharm. 2024;49(4):21-25.
ABSTRACT: Helicobacter pylori, a bacterium that lives in the acidic environment of the stomach, was the third leading cause of cancer deaths globally in 2020. The 2017 American College of Gastroenterology guideline recommends the use of antibiotics, proton pump inhibitors, and (in certain cases) bismuth to treat H pylori. In 2022, vonoprazan-containing regimens, which exhibited noninferiority to and superiority over lansoprazole triple therapy, were approved for H pylori treatment. Although not yet included in the guideline, vonoprazan-containing regimens are a promising alternative because of their efficacy and relative ease of administration. Successful H pylori eradication relies on patient-specific treatment, adherence, and proactive management of antibiotic resistance that comprehends the evolving landscape of therapeutic strategies.
Helicobacter pylori is a spiral-shaped, gram-negative bacterium that thrives in the stomach’s acidic environment. Its prevalence and impact on human health make it a focal point in understanding and addressing gastrointestinal (GI) conditions. In 2015, H pylori infected an estimated 4.4 billion people worldwide. As a major cause of chronic bacterial infections, H pylori is linked to various disorders, ranging from chronic gastritis and peptic ulcers to more serious complications, such as gastric mucosa–associated lymphoid tissue lymphoma and gastric adenocarcinoma.1 Globally, H pylori–related disease ranked as the third leading cause of cancer deaths in 2020, contributing to more than 1 million new cases of gastric cancer and approximately 800,000 deaths.1
Noninvasive diagnostic techniques for H pylori include the urea breath test (UBT), which detects gas produced by the bacterium, and the fecal antigen test, which identifies the bacterium in the stool. To avoid false-negative results, proton pump inhibitors (PPIs), bismuth, and antibiotics must be discontinued at least 2 weeks before testing.1 If left untreated, H pylori infections can, as noted, progress to gastric cancer. Antibiotic resistance and the complexity of medication regimens pose treatment challenges, potentially leading to treatment failure. Treatment should be initiated in symptomatic patients to prevent transmission and mitigate disease-related complications, but evidence is insufficient to support treatment in asymptomatic patients.2
Treatment
The American College of Gastroenterology (ACG) published a treatment guideline for H pylori in 2017.3-5 The guideline divides treatment into three categories. The first category includes recommended first-line treatments (TABLE 1), the second category comprises additional first-line treatment options with less supporting data (TABLE 2), and the third category involves salvage therapy (TABLE 3).3-5 Effectively eradicating H pylori infection requires adherence to multiple agents per day, and emerging resistance to antibiotics is an additional treatment-related challenge. Between 2011 and 2021, clarithromycin-resistant strains of H pylori constituted 31.5% of the 2,660 cases that were evaluated in the United States.1
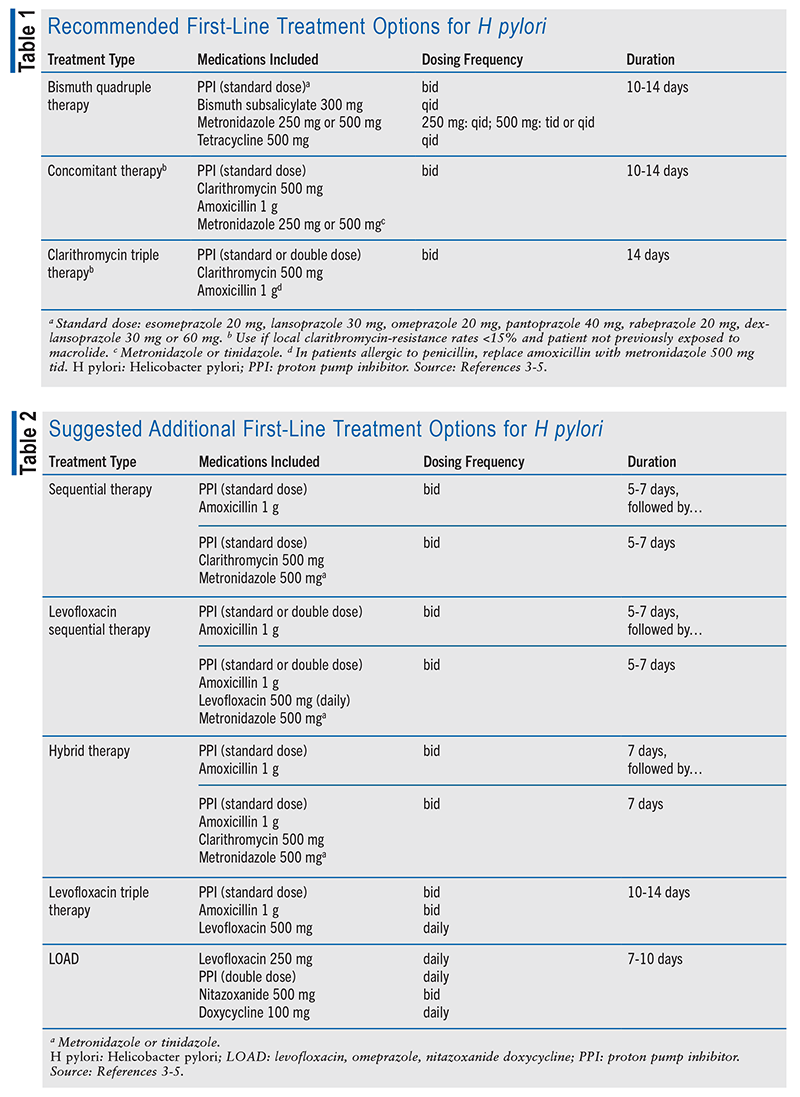
Treatment includes a combination of antibiotics, a PPI, and—depending on the chosen regimen—bismuth. The mechanism behind PPI use for the treatment of H pylori is not entirely understood.6,7 H pylori is neutralophilic, as it survives in a neutral pH of 5.5 to 7.5. One proposed mechanism is that the PPI raises the patient’s gastric pH, weakening the bacterium and contributing to its eradication.6 Another proposed mechanism is that increased gastric pH from a PPI stimulates H pylori growth and more rapid division, resulting in a synergistic effect with antibiotics.7
Recently Approved Therapy
Vonoprazan-containing regimens were approved for the treatment of H pylori in May 2022 and became available in December 2023. Approval was based on results from pHalcon-HP, a large phase III randomized, controlled clinical trial in treatment-naïve adults who tested positive for H pylori and presented with at least one clinical condition. Clinical conditions included dyspepsia (for at least 2 weeks or functional), a recent/new diagnosis of nonbleeding peptic ulcer, a history of peptic ulcer not previously treated for H pylori, and a stable dose of long-term nonsteroidal anti-inflammatory drug treatment. Subjects were randomized into one of three treatment regimens: Voquezna Dual Pak (vonoprazan 20 mg bid/amoxicillin 1 g tid [n = 348]); Voquezna Triple Pak (vonoprazan 20 mg bid/clarithromycin 500 mg bid/amoxicillin 1 g bid [n = 346]); and lansoprazole triple therapy (lansoprazole 30 mg bid/clarithromycin 500 mg bid/amoxicillin 1,000 mg bid [n = 345]). The treatment duration for all participants was 14 days.8,9
Multiple primary endpoints were evaluated with a modified intention-to-treat analysis. For the noninferiority endpoint, Voquezna Triple Pak and Voquezna Dual Pak were compared with lansoprazole triple therapy in subjects without a clarithromycin- or amoxicillin-resistant strain of H pylori. Eradication rates for Voquezna Triple Pak and Voquezna Dual Pak were 84.7% (P <.0001) and 78.5% (P <.01), respectively, versus 78.8% for lansoprazole triple therapy.8,9
For the superiority endpoints, both Voquezna Triple Pak and Voquezna Dual Pak were superior to lansoprazole triple therapy in the overall study population as well as in subjects with a clarithromycin-resistant strain of H pylori. Voquezna Triple Pak had an eradication rate of 80.8% (P <.0003), and Voquezna Dual Pak had a rate of 77.2% (P = .01), versus lansoprazole triple therapy (68.5%). In subjects with a clarithromycin-resistant strain of H pylori, Voquezna Triple Pak and Voquezna Dual Pak achieved eradication rates of 65.8% and 69.6% (both, P <.0001), respectively, versus 31.9% for lansoprazole triple therapy.8,9
Although the pHalcon-HP clinical trial reported all adverse events occurring in at least 2% of subjects, for the sake of relevance, this discussion will focus solely on adverse effects with an incidence of 3% or greater. Diarrhea occurred in 4.0%, 5.2%, and 9.6% of subjects in the Voquezna Triple Pak, Voquezna Dual Pak, and lansoprazole triple therapy groups, respectively. Altered sense of taste was reported in 4.6% and 0.6% of subjects in the Voquezna Triple Pak and Voquezna Dual Pak groups, versus 6.1% in the lansoprazole triple therapy group. Vulvovaginal candidiasis was observed in 3.2%, 2.0%, and 1.4% of subjects in the Voquezna Triple Pak, Voquezna Dual Pak, and lansoprazole triple therapy groups, respectively.7-9
Vonoprazan-containing regimens are not yet included in the current ACG H pylori guidelines, which were last updated in 2017. Vonoprazan has been used in countries outside the U.S. for years, having proved to be both efficacious and safe.10 In the pHalcon-HP clinical trial, Voquezna Triple Pak and Voquezna Dual Pak showed noninferiority and superiority versus lansoprazole triple therapy for the treatment of H pylori.7,8 Vonoprazan-containing regimens hold promise because of their low adverse-effect profile, ease of administration, and high efficacy compared with the standard of care.
Medication Adverse Effects and Management
Before H pylori treatment is initiated, it is critical to screen patients for drug-drug or drug-disease interactions. It is equally important to counsel patients regarding the significance of adherence to the prescribed regimen and the potential adverse effects associated with the medications. TABLE 4 provides a comprehensive overview, detailing the common adverse effects of each medication and essential counseling points.11 This information can serve as a valuable resource for both patients and healthcare professionals in the attainment of a well-informed and effective treatment regimen. Healthcare professionals should monitor for adverse effects during treatment and recommend that the patient take the medications with food to reduce GI effects.
Treatment Challenges
Successful eradication of H pylori depends on patient adherence to therapy and overcoming antibiotic resistance.12 Adherence to the medication regimen can be compromised by a lack of patient education as well as inadequate adverse-effect management. However, a 2022 meta-analysis suggested that enhanced patient education—including telephone calls, direct reminders via messaging applications, and extra information in the form of leaflets and charts—may result in increased eradication rates.13 Burgeoning antibiotic-resistance rates are an argument against empiric therapy with clarithromycin-based regimens, as antibiotic resistance can lead to treatment failure and reinfection.14 The addition of bismuth to a regimen can also help overcome antibiotic resistance.15 Given these challenges, follow-up testing (UBT, fecal antigen test, or biopsy) for proof of eradication is strongly recommended and should be performed at least 4 weeks after completion of initial antibiotic therapy. These tests may be affected by gastric pH, so PPIs and bismuth should be withheld for at least 1 to 2 weeks.5
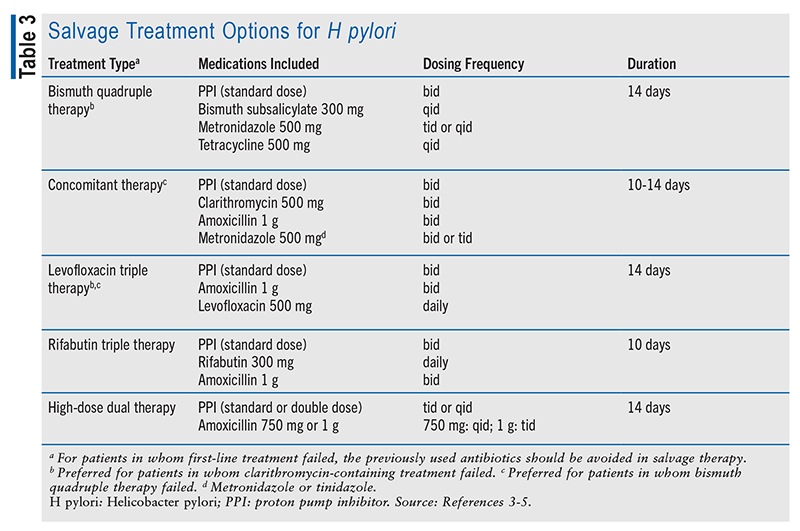
If eradication testing confirms that the initial H pylori treatment failed, it is strongly recommended that the patient be treated with a salvage regimen consisting of a different antibiotic than that previously used. If the patient received a first-line regimen containing clarithromycin, then bismuth quadruple therapy or levofloxacin salvage therapy should be used; a patient who received bismuth quadruple therapy as first-line treatment should be treated with a clarithromycin- or levofloxacin-based salvage regimen.5 Refer to TABLE 3 for more information.
During treatment, patients may encounter challenges such as adverse-effect management, the timing of multiple daily medications and doses, and avoidance of reinfection. Many of these challenges can be effectively managed with patient counseling. Patient education is vital to successful eradication, and it can improve the patient’s understanding of the importance of treatment, leading to better medication adherence.16,17 Adverse effects are common in all H pylori treatment regimens, although many of them can be managed by taking the antibiotics with food. Certain probiotics may reduce the bacterial load in the stomach and can help manage antibiotic-related adverse effects, such as diarrhea.12,13,15 Packaged combination therapy, such as Pylera (bismuth subcitrate/metronidazole/tetracycline), Prevpac (lansoprazole/amoxicillin/clarithromycin), Helidac (bismuth subsalicylate/metronidazole/tetracycline), Talicia (omeprazole/amoxicillin/rifabutin), and Voquezna Triple Pak and Dual Pak, can assist with administration timing and frequency. Finally, encouraging treatment of infected family members and educating patients about frequent handwashing, especially before food preparation, can reduce the risk of infection or reinfection.15
Conclusion
Treatment and eradication of H pylori requires a calculated, multidimensional approach with an emphasis on proper selection of first-line therapy and patient adherence. Treatment selection should be based on patient-specific factors, such as previous antibiotic exposure, type of H pylori, and patient preference. The 2017 ACG H pylori treatment guideline recommends bismuth quadruple therapy, concomitant therapy, or clarithromycin triple therapy for first-line treatment. The recently approved Voquezna Triple Pak and Voquezna Dual Pak are promising options. Follow-up testing to confirm eradication should be performed after therapy completion. In patients with treatment failure, the choice of salvage therapy should exclude all antibiotics used for initial treatment. Improved adherence to the treatment regimen and increased likelihood of successful eradication can be achieved through patient counseling.
REFERENCES
1. Aumpan N, Mahachai V, Vilaichone R. Management of Helicobacter pylori infection. JGH Open. 2022;7(1):3-15.
2. Ansari S, Yamaoka Y. Current understanding and management of Helicobacter pylori infection: an updated appraisal. F1000Res. 2018;7:721.
3. Saleem N, Howden CW. Update on the management of Helicobacter pylori infection. Curr Treat Options Gastroenterol. 2020;18(3):476-487.
4. Randel A. H. pylori infection: ACG updates treatment recommendations. Am Fam Physician. 2018;97(2):135-137.
5. Chey WD, Leontiadis GI, Howden CW, Moss SF. ACG Clinical Guideline: treatment of Helicobacter pylori infection. Am J Gastroenterol. 2017;112(2):212-239.
6. Sachs G, Scott DR, Wen Y. Gastric infection by Helicobacter pylori. Curr Gastroenterol Rep. 2011;13(6):540-546.
7. Scott DR, Sachs G, Marcus EA. The role of acid inhibition in Helicobacter pylori eradication. F1000Res. 2016;5:1747.
8. Voquezna Triple Pak (vonoprazan, amoxicillin, and clarithromycin) and Voquezna Dual Pak (vonoprazan and amoxicillin) product information. Buffalo Grove, IL: Phathom Pharmaceuticals, Inc; May 2022.
9. Chey WD, Mégraud F, Laine L, et al. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: randomized clinical trial. Gastroenterology. 2022;163(3):608-619.
10. Yang C, Li S, Huang T, et al. Effectiveness and safety of vonoprazan-based regimen for Helicobacter pylori eradication: a meta-analysis of randomized clinical trials. J Clin Pharm Ther. 2022;47(7):897-904.
11. Lexicomp Online. Waltham, MA: UpToDate, Inc; 2023. https://online.lexi.com. Accessed February 1, 2024.
12. McFarland LV, Huang Y, Wang L, Malfertheiner P. Systematic review and meta-analysis: multi-strain probiotics as adjunct therapy for Helicobacter pylori eradication and prevention of adverse events. United European Gastroenterol J. 2016;4(4):546-561.
13. Zha J, Li YY, Qu JY, et al. Effects of enhanced education for patients with the Helicobacter pylori infection: a systematic review and meta-analysis. Helicobacter. 2022;27(2):e12880.
14. Mégraud F, Graham DY, Howden CW, et al. Rates of antimicrobial resistance in Helicobacter pylori isolates from clinical trial patients across the US and Europe. Am J Gastroenterol. 2023;118(2):269-275.
15. Elbehiry A, Marzouk E, Aldubaib M, et al. Helicobacter pylori infection: current status and future prospects on diagnostic, therapeutic and control challenges. Antibiotics (Basel). 2023;12(2):191.
16. Driscoll LJ, Brown HE, Harris RB, Oren E. Population knowledge, attitude, and practice regarding Helicobacter pylori transmission and outcomes: a literature review. Front Public Health. 2017;5:144.
17. Stevens VJ, Shneidman RJ, Johnson RE, et al. Helicobacter pylori eradication in dyspeptic primary care patients: a randomized controlled trial of a pharmacy intervention. West J Med. 2002;176(2):92-96.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





