US Pharm. 2024;49(8):HS12-HS16.
ABSTRACT: Influenza significantly impacts pediatric health, with severe complications prevalent among children aged younger than 5 years, especially those with chronic conditions. Annually, influenza results in substantial pediatric hospitalizations and deaths worldwide. Effective management is challenged by the virus’ rapid evolution, leading to vaccine mismatches, and by resistance to antiviral drugs. Prevention through vaccination is crucial, yet it is complicated by factors such as vaccine hesitancy. Diagnostic challenges arise from nonspecific symptoms overlapping with other respiratory infections. Timely and accurate diagnosis, combined with vaccination and public health measures, is essential for managing pediatric influenza and mitigating its impact.
Influenza is an acute respiratory illness that significantly impacts healthcare systems, causing high rates of sickness and death. Influenza belongs to the Orthomyxoviridae family, with types A, B, and C infecting humans; types A and B are the most prevalent.1 Transmission occurs through respiratory droplets, direct contact, and airborne particles.1 In the United States, influenza activity peaks from late fall to early spring. Typically, healthy individuals experience mild-to-moderate symptoms that resolve within days; however, influenza can lead to hospitalizations and fatalities, particularly among vulnerable groups such as children, the elderly, pregnant women, and those with chronic conditions.2 Children aged younger than 5 years are especially prone to hospitalization.3 This article will discuss the impact of influenza on children, including their risk factors, clinical symptoms, diagnosis, and treatment options in hospitals. For pharmacists, understanding these risk factors and managing treatments effectively are vital to prevent severe outcomes in children.
Epidemiology of Pediatric Influenza
On average, influenza accounts for nearly 1 million hospitalizations worldwide in children aged younger than 5 years.4 Up to 10% of the children in the U.S. experience symptomatic influenza annually, resulting in 140,000 to 710,000 hospitalizations and 12,000 to 52,000 deaths each year. For the 2023–2024 flu season, there were 142 pediatric deaths due to complications related to influenza infection.5 Complications vary in children depending on risk factors, which include age and underlying medical conditions. Children aged younger than 5 years—especially children aged younger than 2 years—are at the highest risk of influenza complications.2
Other risk factors of influenza complications include4-7:
• Chronic respiratory illness (e.g., asthma)
• Chronic heart disease, unstable
• Neurologic/neurodevelopmental conditions
• Immunosuppression
• Morbid obesity
• Long-term aspirin therapy for Kawasaki disease
• Repair of congenital heart disease
• HIV.
Influenza can lead to various complications in pediatric patients, ranging from minor to severe life-threatening conditions, including death. Complications may affect a variety of systems, including neurologic, respiratory, cardiovascular, HEENT (head, eyes, ears, nose, throat), and musculoskeletal.4 Among the complications leading to hospitalizations, respiratory problems occur most commonly in children.4,6 Secondary bacterial infections, often caused by Streptococcus pneumoniae and Staphylococcus aureus, may occur. Pneumonia is a significant concern and may affect up to 22% of pediatric patients with influenza, potentially leading to hospitalization and respiratory failure.6 Acute otitis media is frequently observed in 10% to 50% of patients, manifesting within 4 days of influenza infection.4
Additionally, influenza can lead to rare effects, including nonrespiratory complications (e.g., myositis) that may progress to severe conditions such as rhabdomyolysis and acute kidney injury.4 Neurologic complications include febrile and nonfebrile seizures, encephalopathy, and Guillain-Barré syndrome. Cardiovascular conditions such as myositis, sepsis-like syndrome, myocarditis, and pericarditis are rare but may be fatal in children. The likelihood of influenza-related complications and the susceptibility to further infections may be influenced by comorbidities.3,4 These factors serve as important indicators for healthcare professionals to recognize, helping to mitigate morbidity and mortality risks.
A recent study investigated how comorbidities affect hospitalizations for influenza in pediatric patients. The study focused on children with conditions such as heart disease, malignancies (primarily leukemia and lymphoma), and endocrine/metabolic disorders (e.g., obesity, diabetes, and thyroid issues), noting that these can weaken immune responses and increase vulnerability to infections such as pneumonia or meningitis.7 The results showed that 80% of hospitalized children with influenza had comorbidities, with asthma, neurodevelopmental disorders, and gastrointestinal disorders being most common. The study found a higher hospitalization rate among male (65%) compared with female (36%) children. Children with comorbidities were nearly seven times more likely to be hospitalized, with diabetes increasing the risk the most—up to 15 times. Moreover, the type of comorbidity influenced the length of hospital stay; for example, children with heart disease stayed an average of 10 days. The majority of these hospitalizations were among white children, with a noted discrepancy in hospitalization rates among Hispanic and African American children, attributed to socioeconomic differences.
Clinical Presentation and Diagnosis in Hospitalized Children
The clinical presentation of pediatric influenza often differs from that in adults, primarily in symptoms and their duration. Children are particularly vulnerable due to their still-developing immune system, which is not fully capable of producing antibodies until around age 4 years.4,6 This lack of mature immunity makes children frequent targets and carriers of the virus during community outbreaks. Diagnosis primarily relies on observing symptoms, although the Infectious Diseases Society of America guidelines recommend using reverse-transcription polymerase chain reaction to detect influenza antigens in hospitalized patients.4,8 Common symptoms include fever (temperature >100.3° F), chills, cough, sore throat, and malaise.4,6
Pharmacologic Management of Influenza in Pediatric Inpatients
Treating influenza is challenging due to the rapid evolution of the virus, leading to a greater focus on prevention rather than treatment. The main pharmacologic options include neuraminidase inhibitors, such as oseltamivir, zanamivir, peramivir, and baloxavir.3,10 Oseltamivir is the most commonly used agent. These medications often cause gastrointestinal side effects, including nausea, vomiting, and diarrhea (see TABLE 1).
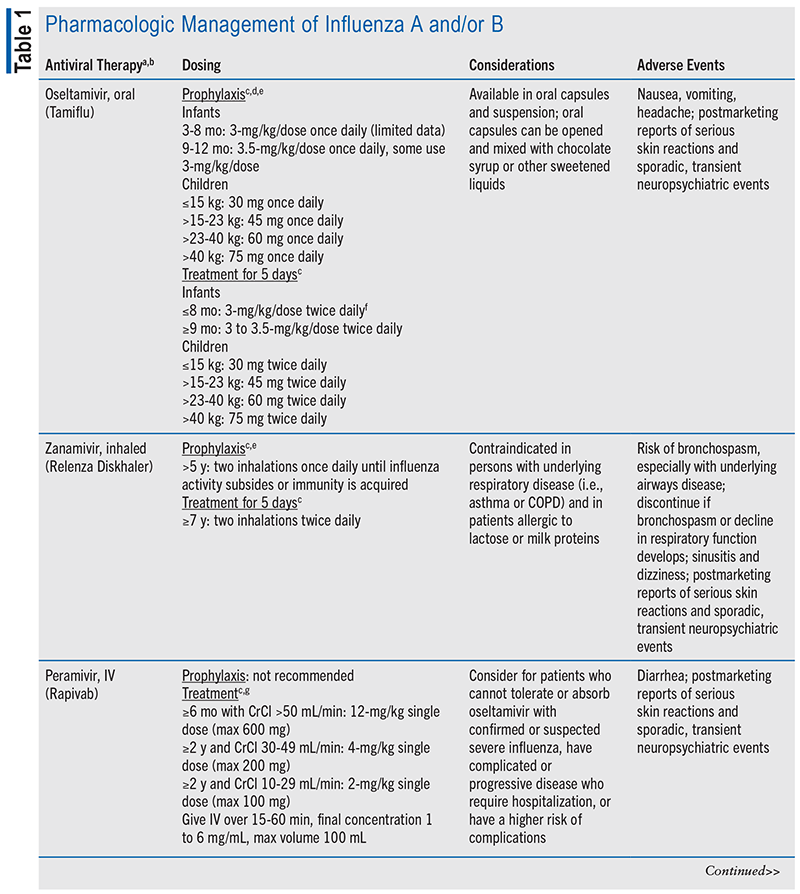
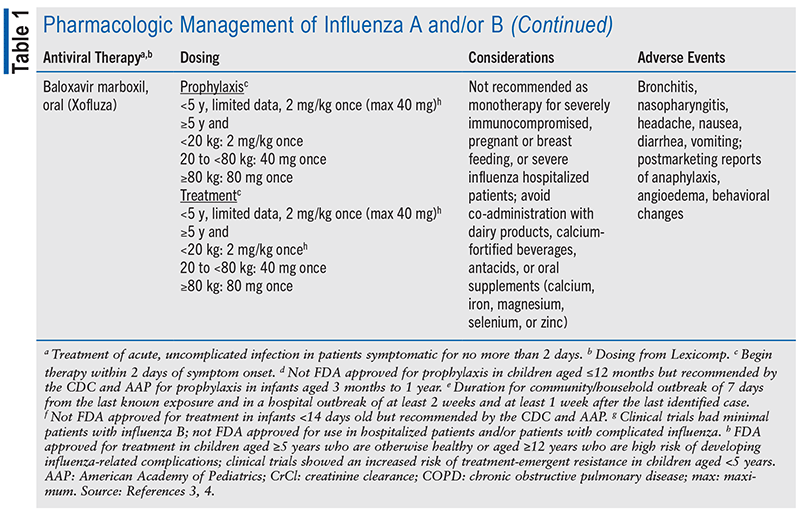
The safety and efficacy of neuraminidase inhibitors in children remain under debate due to limited phase III trial data. Much of the existing data focuses on adults, with scant information specific to pediatric use. Research found that administering these drugs within 48 hours of diagnosing influenza in children significantly improves survival rates compared with delayed treatment (odds ratio 0.36; 95% CI, 0.16-0.84).9 Furthermore, another study showed that children receiving oseltamivir within 24 hours of hospital admission experienced an 18% reduction in the duration of their hospital stay, although it did not affect readmission rates, mortality, or length of pediatric intensive care unit stay.11 Despite these uncertainties, neuraminidase inhibitors are currently the most effective treatments available for reducing hospitalizations due to influenza in children.
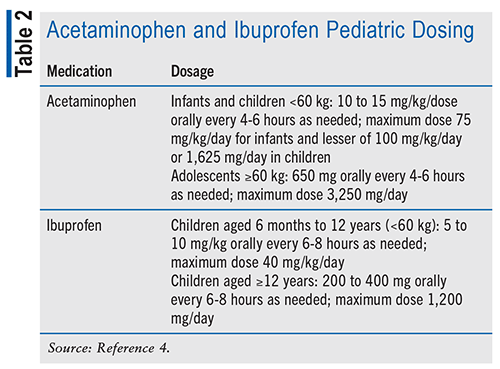
Supportive care for children with influenza includes using ibuprofen and acetaminophen to manage fever and discomfort, alongside rest, fluids, and proper nutrition.3,4,6 While not all children will experience secondary complications from influenza, it is important to be prepared to address them if they occur. Treatment varies depending on the specific complication, with ibuprofen and acetaminophen commonly used to alleviate symptoms.4 See TABLE 2 for acetaminophen and ibuprofen pediatric dosing.
Infection Control and Prevention in Hospital Settings
Stewardship and prevention of infections in the hospital setting focus on proper treatment and robust measures to control the potential spread of the infection beyond “patient zero.” This includes meticulous adherence to hygiene protocols, routine use of personal protective equipment, and effective isolation procedures. Prevention also involves comprehensive management of comorbidities, which can increase susceptibility to infections, and the promotion of annual vaccination campaigns to guard against new strains of the influenza virus. Additionally, educating hospital staff, patients, and visitors about preventive practices plays a critical role in minimizing the risk of hospital-acquired infections.
Although influenza vaccines may not prevent all cases of infection, they offer significant benefits, such as protection against severe consequences of illness and reducing the burden on healthcare resources.2 Currently, trivalent or quadrivalent vaccines are available and recommended for children aged 6 months and older, as endorsed by the American Academy of Pediatrics and the World Health Organization.3,12,13 The vaccine effectiveness for prevention of any acute respiratory illness requiring hospitalization was 52% to 61% for the 2023–2024 influenza season in children aged 6 months to 17 years.14 A study conducted in the U.S. over four influenza seasons from 2010 to 2014 analyzed vaccination rates among pediatric influenza-related deaths. It found that 26% of the 291 children who died had been vaccinated at least 14 days before illness onset.15 Furthermore, 53% of these deaths occurred in children with high-risk medical conditions.15 Another study found that the influenza vaccine significantly reduced hospitalizations and emergency department visits among children, demonstrating 41% effectiveness against hospitalizations and 51% effectiveness against emergency department visits.16 This highlights the vaccine’s role in mitigating severe influenza outcomes and underscores the importance of pediatric vaccination during influenza seasons, especially given the prevalence of various influenza strains.
Challenges and Considerations in Pediatric Influenza Management
Managing pediatric influenza involves multiple challenges and requires a coordinated approach to ensure effective prevention and treatment. One major challenge is the rapid evolution of the virus, leading to antigenic drift that can cause mismatches between the vaccine strains and circulating strains, thus reducing vaccine effectiveness.1 Notably, during the 2014–2015 influenza season, mismatch between the vaccine and the predominant circulating strain (H3N2 virus) resulted in vaccine effectiveness of only 19%.17 This makes both prevention through vaccination and the timely diagnosis of influenza critical, especially for high-risk groups such as children aged younger than 5 years, particularly those aged younger than 2 years, and those with chronic health conditions such as asthma or diabetes. Diagnosis is complicated by influenza’s nonspecific symptoms, which overlap with other respiratory infections, necessitating prompt and accurate testing to initiate early treatment.
Another significant concern is the resistance to key antiviral medications such as oseltamivir and zanamivir, which are used to treat severe cases. Resistance to oseltamivir has been documented, especially in the H1N1 influenza A virus.18 The H275Y mutation in the neuraminidase protein significantly reduces the virus’ susceptibility to oseltamivir, causing a 400-fold decrease in effectiveness compared with wild-type viruses. This mutation became widespread during the 2007–2008 influenza season, leading to high resistance rates globally. However, since the emergence of the A/H1N1pdm09 strain in 2009, oseltamivir resistance has remained relatively low, with less than 3% of cases showing resistance.17 Zanamivir resistance is less common but has been observed, particularly in specific populations such as immunocompromised individuals and critically ill patients. Mutations in A/H3N2 viruses and in influenza B viruses have been linked to reduced susceptibility to zanamivir. These mutations often result in high-level resistance, although they are typically identified in laboratory settings rather than clinical samples.18,19 Continued surveillance and monitoring are essential to detect and manage antiviral resistance.
Monitoring resistance patterns is essential to guide effective treatment decisions. Additionally, maintaining high vaccination rates among children is complicated by factors such as vaccine hesitancy and access issues. The management of pediatric influenza is further complicated by potential coinfections with other pathogens, which can complicate the clinical management and diagnosis strategies.
Conclusion
Effective treatment and prompt identification of influenza outbreaks are critical in managing the health of pediatric populations. Given their vulnerability, vaccination and careful management of comorbid conditions are essential. Failing to adopt these strategies could lead to severe consequences as new influenza virus strains emerge. Therefore, it is imperative that healthcare providers remain vigilant and committed to delivering the best possible care to this sensitive group, adapting to evolving challenges with proactive public health strategies.
REFERENCES
1. Lampejo T. Influenza and antiviral resistance: an overview. Eur J Clin Microbiol Infect Dis. 2020;39(7):1201-1208.
2. World Health Organization. Pandemic Influenza Risk Management: A WHO Guide to Inform and Harmonize National and International Pandemic Preparedness and Response. Geneva: World Health Organization; 2017.
3. Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2023-2024. Pediatrics. 2023;152(4):e2023063773.
4. Wolf RM, Antoon JW. Influenza in children and adolescents: epidemiology, management, and prevention. Pediatr Rev. 2023;44(11):605-617.
5. CDC. Weekly U.S. influenza surveillance report. www.cdc.gov/flu/weekly/index.htm. Accessed April 13, 2024.
6. Kondrich J, Rosenthal M. Influenza in children. Curr Opin Pediatr. 2017;29(3):297-302.
7. Mylonakis SC, Mylona EK, Kalligeros M, et al. How comorbidities affect hospitalization from influenza in the pediatric population. Int J Environ Res Public Health. 2022;19(5):2811.
8. Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infect Dis. 2019;68(6):e1-e47.
9. Louie J, Yang S, Samuel MC, et al. Neuraminidase inhibitors for critically ill children with influenza. Pediatrics. 2013;132(6):e1539-1545.
10. CDC. Influenza antiviral medications: summary for clinicians. www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed May 5, 2024.
11. Kiso M, Mitamura K, Sakai-Tagawa Y, et al. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet. 2004;364(9436):759-765.
12. Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2023-2024. Pediatrics. 2023;152(4):e2023063772.
13. WHO. Recommended composition of influenza virus vaccines for use in the 2024-2025 northern hemisphere influenza season. www.who.int/publications/m/item/recommended-composition-of-influenza-virus-vaccines-for-use-in-the-2024-2025-northern-hemisphere-influenza-season. Accessed May 5, 2024.
14. Frutos AM, Price AM, Harker E, et al. Interim estimates of 2023–24 seasonal influenza vaccine effectiveness—United States. MMWR Morb Mortal Wkly Rep. 2024;73:168-174.
15. Flannery B, Reynolds SB, Blanton L, et al. Influenza vaccine effectiveness against pediatric deaths: 2010-2014. Pediatrics. 2017;139(5):e20164244.
16. Campbell AP, Ogokeh C, Lively JY, et al. Vaccine effectiveness against pediatric influenza hospitalizations and emergency visits. Pediatrics. 2020;146(5):e20201368.
17. CDC. Past seasons’ vaccine effectiveness estimates. Influenza (flu). www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html. Accessed May 13, 2024.
18. Smyk JM, Szydłowska N, Szulc W, Majewska A. Evolution of influenza viruses—drug resistance, treatment options, and prospects. Int J Mol Sci. 2022;23(20):12244.
19. Li TC, Chan MC, Lee N. Clinical implications of antiviral resistance in influenza. Viruses. 2015;7(9):4929-4944.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






