US Pharm. 2014;39(7):HS2-HS6.
ABSTRACT: Respiratory viruses are increasingly recognized as a cause of community-acquired pneumonia in both children and adults. Oseltamivir is recommended for the treatment of influenza virus in the inpatient setting, while ribavirin should be considered for the treatment of respiratory syncytial virus in selected infants and children. Pharmacists are in a key position to actively work with healthcare personnel and providers to choose the most appropriate antiviral regimen for a patient infected with a respiratory virus; to ensure that appropriate infection control measures are followed; and to provide recommendations for effective steps to prevent the spread of infection.
As studies have shown that viruses are the most common cause of community-acquired pneumonia (CAP) in children and are responsible for up to one-third of cases of CAP in hospitalized adults, clinicians should consider a viral etiology in the evaluation of a patient with a respiratory tract infection.1 ,2 Advances in molecular technology have led to the discovery of new respiratory viruses and the increased ability of clinical laboratories to detect their presence. Early diagnosis of a respiratory virus is important to ensure that patients receive the most appropriate treatment as soon as possible, that the use of unnecessary antibiotics is avoided, and that suitable infection control measures are used.1 ,3 This article reviews common respiratory viral pathogens and recommended measures for the treatment and prevention of infections.
EPIDEMIOLOGY
The routine use of vaccines against Streptococcus pneumoniae and Haemophilus influenzae has led to an increasing number of respiratory tract infections caused by viruses.1 The majority of cases of CAP in children are caused by respiratory syncytial virus (RSV), influenza virus, and rhinovirus.1 ,4 Influenza virus and RSV are the most commonly detected viruses in adults hospitalized with CAP.1
It is important to note that there is a high rate of co-infection with viruses and bacteria, with prospective studies of patients with CAP showing that up to one-third of adults and children have more than one identified infecting pathogen.1 Certain viral infections (including influenza virus, RSV, parainfluenza viruses, adenovirus, and rhinoviruses) may also predispose patients to secondary bacterial infections, potentially resulting in more severe disease.1 ,5
DIAGNOSIS
Molecular tests, including nucleic acid amplification assays or multiplex assays that use polymerase chain reaction (PCR) or reverse transcriptase PCR for genomic amplification, are commonly used to evaluate patients suspected of having a respiratory virus and should routinely be used to evaluate children with CAP.3 ,6 Advantages to these tests include a higher sensitivity and faster turnaround time than previously used methods of tissue culture or antigen-based assay.3 Antigen-based rapid influenza diagnostic tests are frequently used by clinicians, since they provide results in <30 minutes. However, the sensitivity of these tests varies, and false-negative test results are common.7 ,8 Rapid diagnostic assays are also available for detection of RSV antigen in nasopharyngeal specimens and are considered to be generally reliable in infants and young children. Due to lower concentrations of RSV shed by older children and adults, these tests have a lower sensitivity in these populations.9
INFECTION CONTROL
It is important to take appropriate precautions to prevent transmission of respiratory viruses in the healthcare setting, as indicated in TABLE 1.4 In addition, there are specific recommendations to prevent the spread of certain viral pathogens. To prevent the spread of RSV and parainfluenza in the healthcare setting, it is recommended that healthcare providers use contact precautions (i.e., place patient in a single room or cohort, only move patient outside the room if medically necessary, wear gloves and gown upon entering room, wear masks and eye protection as needed) in addition to standard precautions.4

To prevent the spread of influenza virus or rhinovirus in the healthcare setting, it is recommended that healthcare providers use droplet precautions (i.e., place patient in a single room or cohort, only move patient outside the room if medically necessary and have patient wear a mask and use respiratory hygiene, wear a surgical mask if close contact with patient is anticipated) in combination with standard precautions.4 ,10 In addition, it is recommended that healthcare personnel wear an N95 respirator mask during procedures that may generate aerosols in order to prevent the spread of influenza virus. To prevent the spread of adenovirus, providers should use droplet precautions in combination with contact precautions.4 ,10
MANAGEMENT OF RESPIRATORY VIRAL INFECTIONS
Antiviral agents for the treatment of respiratory viruses, as well as measures to prevent the acquiring or spread of infection, are included in TABLE 2.11
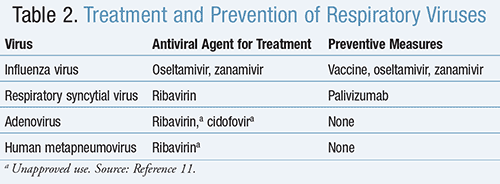
Influenza Virus
Treatment: Antivirals of the neuraminidase inhibitor class that are recommended for the treatment and chemoprophylaxis of influenza virus are included in TABLE 3.7,12-14 Neuraminidase inhibitors may improve outcomes among hospitalized patients by decreasing mortality, complications such as pneumonia or respiratory failure, rate of ICU admission, hospital length of stay, and duration of symptoms.1 ,12 In terms of which neuraminidase inhibitor is preferred, oseltamivir has typically been used in the inpatient setting, since there is a lack of data on the use of inhaled zanamivir in patients with severe influenza.14,15 It is recommended that the clinician consider influenza virus as the etiology of infection in any febrile patient who is hospitalized for a respiratory illness during influenza season.12
Antivirals should be empirically started as early as possible (ideally within 48 hours of the onset of symptoms) for any patient with confirmed or suspected influenza who is hospitalized, has progressive, severe, or complicated illness, or is at higher risk for complications of influenza, without waiting for confirmation of diagnosis from laboratory results.1 ,12,14 If the 48-hour window of time is missed, there is also benefit to starting antiviral therapy as late as 5 days after the onset of symptoms.1,7,12 Antiviral therapy may be continued if there is a high suspicion of influenza virus even with a negative rapid influenza test, since its lower sensitivity may give a false-negative result in a patient with true infection.1 In addition, severely ill patients should be closely monitored for the development of secondary bacterial pneumonia.7
IV zanamivir is available as an investigational agent for the treatment of severe pneumonia and may be obtained by enrollment in an ongoing clinical trial or from an emergency investigational new-drug protocol request to the manufacturer. IV zanamivir should be considered for use in the treatment of patients who cannot tolerate or absorb oral oseltamivir because of gastric stasis, malabsorption, or gastrointestinal (GI) bleeding. IV zanamivir may also be considered for severely ill patients with oseltamivir-resistant virus, which is not common, but has been reported in immunocompromised patients.12 ,15
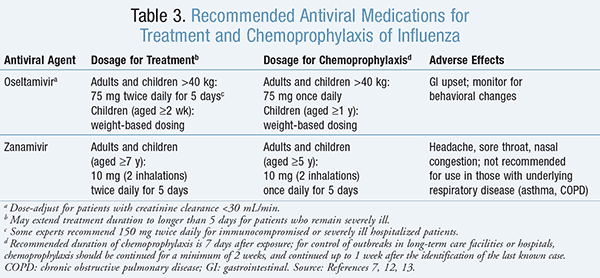
Prevention: Infection control measures, vaccination, and antiviral medications may be used to prevent the spread of influenza virus in the institutional setting. It is important for healthcare workers to receive an annual influenza vaccine prior to the start of influenza season in order to protect themselves from infection, minimize missed days of work, and prevent transmission of influenza to susceptible patients, including those who are at high risk of complications.4 ,16 Antiviral medications should not be considered as a substitute for vaccination, but may be used adjunctively as chemoprophylaxis (TABLE 3). Antiviral chemoprophylaxis can be used for those who are exposed to an infectious person, are at high risk of complications of influenza, and have not yet achieved immunity from vaccination (since it takes approximately 2 weeks to develop a protective antibody response after vaccination) ;14 are severely immunodeficient (since they may have an inadequate antibody response to the influenza vaccine); or are at high risk of complications of influenza but have a contraindication to the influenza vaccine.
In addition, chemoprophylaxis is recommended to control outbreaks of influenza in an institutional setting. Since chemoprophylaxis is recommended to be started as early as possible, it is recommended for institutions to have preapproved physician orders or a plan to obtain orders on short notice so as to expedite the start of therapy.7 ,12
Respiratory Syncytial Virus
Treatment: Ribavirin is the only antiviral agent currently approved for the treatment of RSV in infants and children. The American Academy of Pediatrics recommends against the routine use of ribavirin in children with bronchiolitis, but states that it should be considered in those with documented RSV who have or are at risk of severe disease (such as those who are immunocompromised or have significant cardiopulmonary disease) .17 The use of ribavirin is not recommended for all patients because of its marginal benefit (some clinical trials have shown some benefit with its use while others have not), high cost, potential health risks for caregivers, and cumbersome delivery requirements.17
Aerosolized ribavirin is the preferred route of administration (over IV or oral formulations) in order to minimize its systemic toxicities.18 Use of ribavirin requires specially trained personnel and specialized equipment for administration, with the agent typically administered via a small particle aerosol generator at a concentration of 20 mg/mL for 12 to 18 hours per day for 3 to 7 days. Since ribavirin is potentially mutagenic and is classified as Pregnancy Category X, healthcare workers who are pregnant should be counseled about potential risks of exposure and risk reduction strategies. In addition, respiratory function should be monitored carefully in those receiving ribavirin who have asthma, and it should be used with caution in patients who require mechanical ventilation, as drug precipitation may interfere with equipment function.13
Although RSV is increasingly recognized as a cause of respiratory disease in the elderly (aged >65 years) and in high-risk adults (immunocompromised or with chronic heart or lung disease), especially among those residing in a long-term care facility, there are no specific recommendations regarding the use of ribavirin in adults.19-21 Most immunocompetent adults with RSV infection are managed with agents targeting relief of symptoms, including fluids and supplemental oxygen. Bronchodilators may be used in severe cases, and corticosteroids are an option for those with asthma or chronic obstructive pulmonary disease (COPD) who have wheezing.19 Ribavirin has been used off-label (alone or in combination with IV immunoglobulin or palivizumab) at a dosage of 6 g/day via various dosing schedules for the treatment of RSV in severely immunocompromised adults, defined as those who have undergone heart or lung transplants or hematopoietic stem cell transplantation (HSCT) .13,18 Ribavirin is not typically used in other adult populations because the benefits do not outweigh its costs and risks of use.1
Prevention: There is no vaccine currently available for the prevention of RSV in either infants or adults.19 Palivizumab, a monoclonal antibody that prevents entry of RSV into the host cell and fusion of RSV-infected cells, is indicated for the prevention of serious lower respiratory tract disease caused by RSV in pediatric patients at high risk for RSV disease.9 ,22 Prophylaxis with palivizumab may be used in premature infants or children aged 24 months with underlying chronic pulmonary or congenital heart disease, according to specific criteria as indicated at www.cdc.gov/rsv/clinical/prophylaxis.html.9,20 When given monthly at a dosage of 15 mg/kg IM prior to the start of and during RSV season, which is generally between November and April, this agent decreases the need for RSV-related hospitalization.9
There have also been a few case reports describing the use of palivizumab (in addition to infection control measures) to control outbreaks of RSV in neonatal ICUs, but there are no controlled trials that have evaluated this practice.4
Other Viruses
The treatment of other respiratory viruses is not as well defined. There are a few case reports regarding the use of oral or IV ribavirin for the treatment of viral pneumonia caused by human metapneumovirus ( hMPV), parainfluenza virus, or adenovirus in severely immunocompromised patients, and the use of cidofovir for the treatment of adenovirus in those severely immunocompromised.1
ROLE OF THE PHARMACIST
Pharmacists can play an integral role in the management of respiratory viral infections. They should work with clinicians to select the most appropriate antiviral regimen for a particular patient and can work with the healthcare facility to ensure that appropriate infection control measures are followed. Pharmacists can assist in the selection of the most appropriate antiviral agents, monitor for adverse effects, and provide recommendations for preventive measures. Pharmacists are in a key position to actively work with providers to ensure the proper management of respiratory viral infections and to prevent their spread in the workplace.
REFERENCES
1. Pavia A. What is the role of respiratory viruses in community acquired pneumonia; what is the best therapy for influenza and other viral causes of CAP? Infect Dis Clin North Am. 2013
;27:157-175.
2. Jennings L, Anderson T,
Beynon K, et al. Incidence and characteristics of viral community-acquired pneumonia in adults. Thorax. 2008
;63:42-48.
3. Beck E,
Henrickson K. Molecular diagnosis of respiratory viruses. Future
Microbiol. 2010
;5:901-916.
4.
Goins W, Talbot H, Talbot T. Health care-acquired viral respiratory diseases. Infect Dis Clin N Am. 2011
;25:227-244.
5. McCullers J. Insights into the interaction between influenza virus and pneumococcus. Clin
Microbiol Rev. 2006
;19:571-582.
6. Bradley J,
Byington C, Shah S, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011
;53:e25-e76.
7. CDC. Influenza antiviral medications: summary for clinicians.
www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm. Accessed March 14, 2014.
8. Chan M, Lee N,
Ngai K, et al. Clinical and
virologic factors associated with reduced sensitivity of rapid influenza diagnostic tests in hospitalized elderly patients and young children. J Clin
Microbiol. 2014
;52:497-501.
9. American Academy of Pediatrics.
Respiratory syncytial virus. In: Pickering LK, ed. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2012.
10. Siegel JD,
Rhinehart E, Jackson M,
Chiarello L; the Healthcare Infection Control Practices Advisory Committee. 2007 guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings.
Am J Infect Control. 2007
;35(10 suppl 2):S65-S164.
11.
Ruuskanen O, Lahti E, Jennings L, et al. Viral pneumonia. Lancet. 2011
;377:1264-1275.
12. Fiore AE, Fry A, Shay D, et al; CDC. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR
Recomm Rep. 2011
;60:1-25.
13.
Lexi-Comp Online. Hudson, OH:
Lexi-Comp,
Inc; 2014.
www.lexi.com. Accessed March 14, 2014.
14.
Mandell LA,
Wunderink RG,
Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007
;44(suppl 2):S27-S72.
15. CDC. Intravenous influenza antiviral medications and CDC considerations related to investigational use of intravenous
zanamivir for
2013-2014 influenza season.
www.cdc.gov/flu/professionals/antivirals/intravenous-antivirals.htm. Accessed March 14, 2014.
16. CDC. What you should know for the 2013-2014 influenza season.
www.cdc.gov/flu/about/season/flu-season-2013-2014.htm. Accessed March 14, 2014.
17. American Academy of Pediatrics.
Diagnosis and management of bronchiolitis. Pediatrics. 2006
;118: 1774-1793.
18.
Hynicka L, Ensor C. Prophylaxis and treatment of respiratory syncytial virus in adult
immunocompromised patients. Ann
Pharmacother. 2012
;46:558-566.
19. Walsh E. Respiratory syncytial virus
infection in adults.
Semin
Respir
Crit Care Med. 2011
;32:423-432.
20. CDC. Respiratory syncytial virus (RSV): prophylaxis and high-risk groups.
www.cdc.gov/rsv/clinical/prophylaxis.html. Accessed March 14, 2014.
21.
Falsey A. Respiratory syncytial
virus: a global pathogen in an aging world. Clin Infect Dis. 2013
;57:1078-1080.
22.
Synagis (palivizumab) package insert. Gaithersburg, MD:
MedImmune
; April 2013.
To comment on this article, contact rdavidson@uspharmacist.com.






