US Pharm. 2020;45(11):17-22.
ABSTRACT: In the United States, diabetes mellitus remains a significant risk factor for the development of and death from cardiovascular disease (CVD). While numerous medications have been approved by the FDA for the treatment of hyperglycemia, their cardiovascular safety has been questioned and is the subject of ongoing CV outcome trials (CVOTs). Since 2008, CVOT results have varied from demonstration of noninferior to superior safety compared with placebo. Some agents in particular have raised additional concerns regarding specific conditions, including heart-failure risk. Current literature addressing various drug classes’ effects on CV safety is summarized below. As clinicians, pharmacists can utilize available evidence as well as emerging CVOT data to ensure appropriate treatment for patients with or at high risk for CVD.
In the United States, at least 68% of individuals aged 65 years and older with diabetes die from some form of cardiovascular disease (CVD).1 Prevention of macrovascular complications such as coronary heart disease, stroke, and peripheral vascular disease is of crucial importance in the treatment of patients with diabetes. However, despite a decrease in diabetes-related CV complications over the past 2 decades, patients with diabetes have a two- to four-fold increased risk of death from heart disease compared to those without diabetes.1
Early examination of the CV risks and benefits of common medications revealed mixed results depending on the agent tested. Metformin appears to have potential atherosclerotic cardiovascular disease (ASCVD) benefit, based upon the results of the landmark UK Prospective Diabetes Study.2 This trial showed that metformin significantly decreased all-cause mortality as well as diabetes-specific mortality (defined as death from myocardial infarction [MI], stroke, peripheral vascular disease, kidney disease, hypoglycemia or hyperglycemia, or sudden death) in newly diagnosed, overweight patients with type 2 diabetes (T2D); however, these patients were at low CVD risk at baseline (aged 25-65 years with newly diagnosed T2D and excluded if they had experienced MI within the past year, had experienced more than one major vascular event, or had current congestive heart failure).2 These benefits persisted after an additional 10-year follow-up period.3 A 2005 Cochrane review of 29 randomized trials that compared metformin to lifestyle modification or to other antiglycemic agents (including sulfonylureas and thiazolidinediones [TZDs]) showed a benefit of metformin monotherapy across multiple outcomes such as glycemic control, all-cause mortality, and incidence of MI, with obese patients experiencing greater benefit compared with overweight patients.4
TZDs, studied in various trials, have a less straightforward ASCVD benefit. In 2005, the PROactive study published results of pioglitazone’s effects on macrovascular outcomes.5 While its primary endpoint (a composite including all-cause mortality, nonfatal MI, stroke, acute coronary syndrome, leg revascularization, and major leg amputation) did not reach significance, a main secondary composite endpoint (all-cause mortality, nonfatal MI, and stroke) showed a significant benefit with pioglitazone (hazard ratio [HR] 0.84; 95% CI 0.72-0.98; P = .027). However, heart failure requiring admission was increased in the pioglitazone group, likely due to increased fluid retention seen with the TZD class. Further complicating the CV conclusions regarding TZDs, a 2007 meta-analysis of rosiglitazone reported a 43% increase in MI incidence and a 64% increase in death from CV causes.6
Since these first trials, numerous antidiabetic agents have been approved in the U.S.; yet, the FDA has grappled with uncertainty surrounding their CV safety. In 2008, due in part to the aforementioned 2007 meta-analysis, the FDA stated in a guidance document that all new antidiabetic therapies in T2D should demonstrate no unacceptable increase in CV risk.7 To comply with this guidance, the era of CV outcomes trials (CVOTs) began, increasing the cost and the scope of research required for the approval of new antidiabetic agents. Of the numerous CVOTs that have been published, results vary widely from noninferiority to superiority of the agent compared to treatment with placebo when assessing CV outcomes. However, some studies have raised additional concerns with specific agents in relation to other CV results, such as worsening of heart failure. Summarized below are major CVOTs that have been published since the issuance of the FDA guidance, which will continue to inform treatment decisions for patients with diabetes with or at high risk for CVD.
DPP-4 Inhibitors
In addition to their glucose-lowering effects, potential mechanisms for dipeptidyl peptidase-4 (DPP-4) inhibitors’ CV effect include increased ischemia tolerance, improved endothelial function, decreased expression of inflammatory cytokines, and decreased platelet aggregation.8 The EXAMINE trial addressed alogliptin’s effects on a traditional MACE (major adverse cardiac events; composite of nonfatal stroke, nonfatal MI, and CV death) outcome, finding that alogliptin was noninferior, but not superior, to treatment with placebo.9 SAVOR-TIMI 53 investigated the same composite outcome for saxagliptin and obtained the same result. However, it was also found that rates of hospitalization for heart failure were significantly increased by 27% with saxagliptin.10
Results from TECOS for sitagliptin, which investigated the drug’s effect on a 4-point MACE outcome, including hospitalization for unstable angina, also demonstrated noninferiority of the DPP-4. The effect on hospitalization for heart failure was also investigated, with results showing no increased risk for those taking sitagliptin.11 Most recently, the CARMELINA study, which addressed the effects of linagliptin, found the drug to be noninferior to placebo regarding the primary MACE outcome as well as hospitalization for heart failure.12 All in all, investigations of DPP-4 medications have revealed consistently noninferior results regarding MACE compared with placebo; however, it is important to consider the differences in these trials in relation to the differing effects on heart-failure hospitalizations.
GLP-1 Receptor Agonists
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) mimic the effects of the incretin hormone GLP-1, which stimulates glucose-dependent insulin release and delays gastric emptying, commonly resulting in weight loss.13 While native GLP-1 is quickly degraded in the body by the enzyme DPP-4, longer-acting pharmacologic therapies allow for once-daily or once-weekly GLP-1 RA administration. As summarized below, several studies have assessed CV outcomes with the use of GLP-1 RAs; however, the precise mechanism for any risk reduction seen with these agents remains unknown. Possibilities include a reduction in hemoglobin A1C, LDL cholesterol, blood pressure, and weight.14 Additionally, it has also been suggested that GLP-1 RAs may independently improve endothelial and platelet function and could also have direct neuroprotective effects.15
The first published major CVOT is the ELIXA trial, which assessed the CV effects of once-daily lixisenatide compared with volume-matched placebo. ELIXA enrolled patients with T2D who had experienced an acute coronary event within 180 days before screening. The primary composite outcome of major adverse cardiac events plus (MACE+), including death from CV causes, nonfatal MI, and hospitalization for unstable angina, was reached in 13.4% of patients in the lixisenatide group and 13.2% of patients in placebo group, demonstrating noninferiority, but not superiority, of lixisenatide compared with placebo.16
Following ELIXA, the LEADER trial assessed CV outcomes in patients using daily liraglutide versus placebo. LEADER enrolled T2D patients with or without concomitant CVD (81% of patients enrolled with established CVD and 19% with CV risk factors). The primary outcome of MACE composite (death from CV causes, nonfatal MI, nonfatal stroke) occurred in 13% fewer liraglutide patients compared with placebo (13% vs. 14.9%), resulting in a statistically significant, superior outcome for liraglutide.17
Shortly following the publication of LEADER, SUSTAIN-6 reported findings for semaglutide. T2D patients with and without established CVD were enrolled. While a significantly lower risk of the composite primary MACE outcome was seen in the semaglutide group compared with placebo, SUSTAIN-6 was not powered to show superiority, and it was therefore concluded that semaglutide is noninferior to placebo regarding MACE outcomes.18 PIONEER 6, a trial examining the oral formulation of semaglutide also showed noninferiority of the GLP-1 RA compared with placebo in regard to CV risk.19
One of the largest CVOTs to date, EXSCEL examined the impact of extended-release (ER) exenatide on CV outcomes. EXSCEL featured wide-ranging eligibility criteria and included T2D patients with or without previous CV events. The primary composite MACE outcome was reached in 11.4% of patients treated with ER exenatide and 12.2% of those treated with placebo (nonsignificant for superiority). Overall, it was concluded that ER exenatide is noninferior to placebo with respect to CV safety but not superior to placebo with respect to efficacy.20
The REWIND trial reported findings for the GLP-1 RA dulaglutide. REWIND enrolled T2D patients with or without established CVD. Despite a relatively low percentage of patients with preexisting CVD compared with previous CVOTs, the primary composite MACE outcome (including death from unknown causes) occurred in 12% of the dulaglutide group and 13.4% of the placebo group. The significant HR (0.88) was determined to be similar for those with or without previous CVD and in those with A1C less than or greater than 7.2%, demonstrating GLP-1 RAs that reduce CV outcomes should be considered in patients across a wide range of A1C values and in those with CV risk factors but without established CVD.14 Overall, the CVOTs investigating the various GLP-1 RA options for treatment of T2D have demonstrated acceptable CV safety across all available medications. While certain trials were able to demonstrate superiority of a GLP-1 RA over placebo (LEADER, REWIND), the lack of head-to-head comparisons of these therapeutic options limits the ability to make definitive conclusions regarding the efficacy of one agent over another, especially considering the differences between the CVOTs regarding median follow-up, number of enrolled patients, statistical power, and proportion of patients with CVD. Generally, the selection of a GLP-1 RA should involve consideration of available evidence in conjunction with relevant patient-specific factors such as CV risk, cost, and patient adherence.
SGLT2 Inhibitors
Sodium-glucose cotransporter-2 (SGLT2) inhibitors work within the proximal convoluted tubule in the nephron, inhibiting SGLT2 and subsequently preventing reabsorption of glucose into the bloodstream and facilitating its removal via urinary excretion.21 Additionally, the administration of SGLT2 inhibitors results in a mild osmotic diuretic effect, as they also reduce sodium and protein reabsorption in the nephron.21 Several hypotheses exist to explain why SGLT2 inhibitors may confer CV and renal protection, as shown in the studies below. Plausible explanations include improved glycemic control, lowering of blood pressure, decrease in intraglomerular pressure, reduction in albuminuria, and improvement in volume overload.22
EMPA-REG was the first CVOT to examine an SGLT2 inhibitor, empagliflozin. EMPA-REG enrolled T2D patients with established CVD. The primary MACE composite occurred in 10.5% of the empagliflozin group and 12.1% of the placebo group, highlighting a significant difference and superior result for empagliflozin over placebo. No significant between-group differences were noted for nonfatal MI or stroke; the primary outcome was mainly driven by a significant decrease in risk of death from CV causes (3.7% vs. 5.9%). Empagliflozin also resulted in a decreased rate of death from any cause and a decrease in hospitalization for heart failure compared with placebo.23
Data for another SGLT2, canagliflozin, were published as the CANVAS Program, a joint analysis of two sister studies, CANVAS and CANVAS-Renal. T2D patients with or without ASCVD were enrolled. The primary MACE composite was found to be significantly less in the canagliflozin group compared with placebo; in addition, hospitalization for heart failure was found to be decreased with canagliflozin, but this result was not considered to be statistically significant.22 While overall rare, an unexpected adverse event of significantly increased amputation risk with canagliflozin also emerged in this trial. Prespecificed renal outcomes, including progression of albuminuria and a renal composite (40% reduction in estimated glomerular filtration rate, need for renal replacement therapy, or death from renal causes), while not statistically significant, showed the potential benefit of canagliflozin that was further studied in the CREDENCE trial. CREDENCE investigated the impact of canagliflozin on T2D patients with albuminuric chronic kidney disease (CKD). The primary composite outcome (end-stage kidney disease, a doubling of serum creatine, or death from renal or CV causes) was found to be 30% lower in the canagliflozin group.24
Finally, recent results from the DECLARE-TIMI 58 trial demonstrated the CV safety of dapagliflozin. T2D patients with or without ASCVD were followed, and it was determined that dapagliflozin is noninferior to placebo with regard to MACE. While dapagliflozin did not result in a significantly lower rate of MACE, it did produce a 17% reduction in rate of hospitalization for or death from heart failure.25 In summary, SGLT2 inhibitors offer various CV benefits that may arise independently of their glucose-lowering effects. Empagliflozin and canagliflozin both offer significant ASCVD benefit, as shown in EMPA-REG and CANVAS trials; however, it is important to consider that EMPA-REG enrolled only secondary prevention patients while CANVAS included a broader population.22,23 While DECLARE-TIMI 58 did not demonstrate superiority of dapagliflozin over placebo for MACE, it did show a reduction in heart-failure hospitalization similar to that seen with empagliflozin or canagliflozin.25
Future Directions
Since publication of FDA guidance in 2008 recommending establishment of CVOTs to assess safety of new antidiabetic therapies, numerous trials have investigated the link between these medications and their effects on MACE. While many trials (such as ELIXA for lixisenatide or TECOS for sitagliptin) established a noninferior relationship of antidiabetic agents compared with placebo, some showed superiority (such as LEADER for liraglutide and EMPA-REG for empagliflozin), and others raised separate concerns about additional CV outcomes, such as increased hospitalization for heart failure (e.g., SAVOR-TIMI 53 for saxagliptin). Ongoing or recently completed trials include those investigating the CV effects of the SGLT2 inhibitor ertugliflozin and the oral formulation of GLP-1 RA semaglutide. In coming years, recently completed trials will be incorporated into current diabetes-treatment guidelines, and additional studies will address increasingly specific patient populations such as those with diabetes and CKD as well as those with heart failure and reduced ejection fraction.26,27
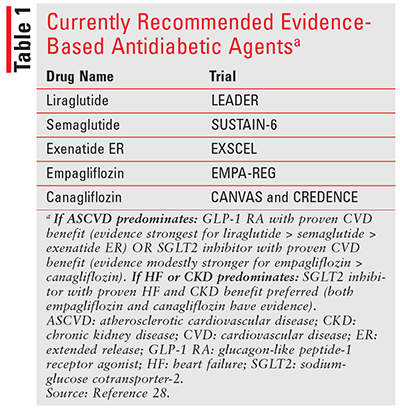
Taken together, the broad CVOTs can be difficult to assimilate; however, some general findings and recommendations influenced by these trials exist to help clinicians improve CV outcomes for patients with diabetes. The most recent guidelines published by the American Diabetes Association continue to recommend an initial regimen of metformin and lifestyle modifications for most patients with A1C levels above their goal.28 However, when considering additional agents beyond metformin, it is important to first consider the patient’s ASCVD status. In a patient with established ASCVD, treatment with an evidence-based GLP-1 RA or SGLT2 inhibitor is recommended (TABLE 1).28 In a patient with established heart failure or CKD, treatment with an evidence-based SGLT2 inhibitor is recommended (TABLE 1).28 As with any other medication choice, patient preference is a key consideration and should at minimum include a discussion of cost, route of administration, and dosing schedule.
Conclusion
In the future, it is likely that further study results and recommendation updates will continue to shape the clinical decision-making process regarding patients with T2D and CVD. Helpful guideline updates could include topics such as the treatment of patients with diabetes at high risk for CVD but without established disease as well as expansion of beneficial treatment options for patients with T2D and heart failure or CKD. As clinicians, pharmacists should continue to work toward optimizing patient outcomes by remaining up-to-date on CV trial results and guideline publications as they become available, utilizing new data to ensure appropriate pharmacologic treatment choices for each patient.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. American Heart Association. Cardiovascular Disease and Diabetes. www.heart.org/en/health-topics/diabetes/why-diabetes-matters/cardiovascular-disease–diabetes. Accessed October 28, 2020.
2. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):854-865.
3. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577-1589.
4. Saenz A, Fernandez-Esteban I, Mataix A, et al. Metformin monotherapy for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(3):CD002966.
5. Dormandy JA, Charbonnel B, Eckland DJA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366(9493):1279-1289.
6. Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356(24):2457-2471.
7. FDA. Guidance for industry: diabetes mellitus–evaluating cardiovascular risk in new antidiabetic therapies to treat type 2 diabetes. www.federalregister.gov/documents/2008/12/19/E8-30086/guidance-for-industry-on-diabetes-mellitus-evaluating-cardiovascular-risk-in-new-antidiabetic. December 2008. Accessed October 28, 2020.
8. Kim N-H, Yu T, Lee DH. The nonglycemic actions of dipeptidyl peptidase-4 inhibitors. Biomed Res Int. 2014;2014:368703.
9. White WB, Cannon CP, Heller SR, et al. Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med. 2013;369(14):1327-1335.
10. Scirica BM, Bhatt DL, Braunwald E, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317-1326.
11. Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373(3):232-242.
12. Rosenstock J, Perkovic V, Johansen OE, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321(1):69-79.
13. Garber AJ. Long-acting glucagon-like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34(Suppl 2):S279-S284.
14. Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet.
2019;394(10193):121-130.
15. Sposito AC, Berwanger O, de Carvalho LSF, Saraiva JFK. GLP-1RAs in type 2 diabetes: mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc Diabetol. 2018;17(1):157.
16. Pfeffer MA, Claggett B, Diaz R, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373(23):2247-2257.
17. Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311-322.
18. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834-1844.
19. Pratley R, Amod A, Hoff ST, et al. Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet. 2019;394(10192):39-50.
20. Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377(13):1228-1239.
21. Kalra S. Sodium glucose co-transporter-2 (SGLT2) inhibitors: a review of their basic and clinical pharmacology. Diabetes Ther. 2014;5(2):355-366.
22. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657.
23. Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128.
24. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306.
25. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347-357.
26. EMPagliflozin outcomE tRial in Patients With chrOnic heaRt Failure With Reduced Ejection Fraction (EMPEROR-Reduced)–Full Text View–ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03057977. Accessed October 28, 2020.
27. A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients With Chronic Kidney Disease–Full Text View–ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03036150. AccessedOctober 28, 2020.
28. American Diabetes Association. Standards of Medical Care in Diabetes-2020 abridged for primary care providers. Clin Diabetes. 2020;38(1):10-38.
To comment on this article, contact rdavidson@uspharmacist.com.





