U.S. Pharm. 2010;35(1):44-45.
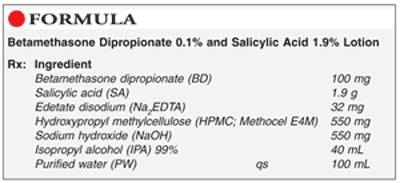
Method of Preparation: Calculate the quantity of each ingredient for the amount to be prepared. Accurately weigh or measure each ingredient. Place ≈30 mL PW in a suitable container. While agitating, slowly add HPMC; mix well. In a separate beaker, dissolve BD in ≈12 to 15 mL IPA. In another container, dissolve NaOH in ≈5 mL PW. Dissolve Na2EDTA in ≈5 mL PW in a different beaker. In another container, dissolve SA in ≈10 mL PW. While mixing, slowly add ≈20 mL IPA to HPMC over ≈15 minutes. To this, add NaOH solution, followed by SA solution and Na2EDTA solution; mix well. Check pH (desired range 4.8-5.3); adjust if necessary. Add BD solution and sufficient PW to final volume; mix well. Package and label.
Use: BD and SA lotion is used to treat selected corticosteroid-responsive dermatoses where a mild keratolytic action is required.
Packaging: Package in tight, light-resistant containers.
Labeling: Shake well before using. Keep out of the reach of children. Discard after 6 months.
Stability: A beyond-use date of up to 6 months may be used.1
Quality Control: Quality-control assessment can include theoretical weight versus actual weight, pH, specific gravity (SG), active drug assay, color, rheologic properties, and physical observations.2
Discussion: BD (C28H37FO7, MW 504.60) occurs as a white to cream-colored, odorless powder; it is insoluble in water and sparingly soluble in alcohol. It is a topical corticosteroid.1
SA (C7H6O3, MW 138.12) occurs as white crystals (usually fine needles) or as a fluffy, white, crystalline powder. It may have a slight yellow or pink tint and a faint, mintlike odor. It is stable in air, slightly soluble in water (1 g in 460 mL; 1 g in 15 mL boiling water), and freely soluble in alcohol (1 g in 3 mL). It should be protected from light.1
Na2EDTA (C10H14N2Na2O8, MW 336.21, edetic acid disodium, disodium ethylenediaminetetraacetate) occurs as an odorless, white, crystalline powder with a slightly acidic taste. It is soluble 1 g in 11 mL water, slightly soluble in 95% ethanol, and practically insoluble in chloroform and ether. The pH of a 1% w/v solution in CO2-free water ranges from 4.3 to 4.7. Na2EDTA also occurs as dihydrate (C10H14N2Na2O8.2H2O, MW 372.20). It melts at 252°C, with some decomposition. It forms a stable soluble complex with calcium. It is generally used topically in concentrations of 0.005% to 0.1%.3,4
HPMC (hypromellose, cellulose, hydroxypropyl methyl ether; Methocel; Pharmacoat) occurs as an odorless and tasteless, white or cream-colored, fibrous or granular powder. It is used as a coating agent, film former, rate-controlling polymer, stabilizing agent, suspending agent, tablet binder, and viscosity-increasing agent. The pH of a 1% aqueous solution ranges from 5.5 to 8.0. HPMC is soluble in cold water--forming a viscous colloidal solution--and practically insoluble in alcohol. Methocel E4M has a nominal viscosity of 4,000 mPa for a 2% w/v aqueous solution at 20°C. It is incompatible with some oxidizing agents.5
NaOH (MW 40.00, caustic soda, soda lye) occurs as dry, very deliquescent, white or almost white sticks, pellets, or fused masses that are hard and brittle. It is strongly alkaline and corrosive and rapidly absorbs moisture and CO2 when exposed to air. It is soluble 1 g in 1 mL water and is freely soluble in alcohol. A 0.01% solution in water has a pH of not less than 11.0. It should be stored in airtight, nonmetallic containers.6,7
IPA (isopropanol) is a clear, colorless, mobile, volatile, flammable liquid with a characteristic odor resembling a mixture of ethanol and acetone, with a bitter taste. It should not be taken orally. IPA boils at 82.4°C and has an SG of 0.786. It is miscible with chloroform, ethanol, glycerin, and water; soluble in acetone; and insoluble in salt solutions. It should be stored in a cool place. IPA is incompatible with oxidizing agents. It may be salted out from aqueous mixtures by the addition of sodium chloride, sodium sulfate, or other salts.8
REFERENCES
1. USP Pharmacists' Pharmacopeia. 2nd ed. Rockville, MD: US Pharmacopeial Convention, Inc; 2008:90,312,1426,1459.
2. Allen LV Jr. Standard operating procedure for performing physical quality assessment of ointments/creams/gels. IJPC. 1998;2:308-309.
3. Thassu D. Edetic acid. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. Washington, DC: American Pharmaceutical Association (APA); 2009:247-250.
4. Shah S, Thassu D. Disodium edetate. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. Washington, DC: APA; 2009:242-244.
5. Rogers TL. Hypromellose. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. Washington, DC: APA; 2009:326-329.
6. Kibbe AH. Sodium hydroxide. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. Washington, DC: APA; 2009:648-649.
7. Reynolds JEF, ed. Martindale: The Extra Pharmacopeia. 30th ed. London, England: Pharmaceutical Press; 1993:1415.
8. McCoy CP. Isopropyl alcohol. In: Rowe RC, Sheskey PJ, Quinn ME, eds. Handbook of Pharmaceutical Excipients. 6th ed. Washington, DC: APA; 2009:346-348.
To comment on this article, contact rdavidson@uspharmacist.com.





