US Pharm. 2021;46(12):58-59.
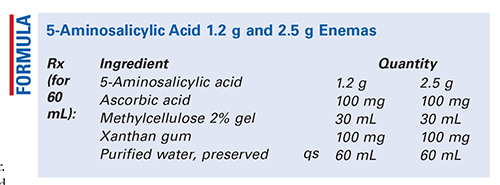
Method of Preparation: Calculate the required quantity of each ingredient for the total amount to be prepared. Accurately weigh or measure each ingredient. Place the 5-aminosalicylic acid (5-ASA) in a glass mortar. Add the ascorbic acid and xanthan gum, and triturate well. In divided portions, add the methylcellulose gel to form a smooth, lump-free dispersion. Add sufficient preserved purified water to final volume in divided portions, and mix until uniform. Package in enema-administration containers and label.
Use: Mesalamine is an anti-inflammatory agent that is used in the treatment of certain gastrointestinal disorders.
Packaging: Package in tight, light-resistant containers.
Labeling: Keep out of reach of children. Discard after ____ [time period]. For rectal use.
Stability: A beyond-use date of up to 30 days may be used for this preparation.1
Quality Control: Quality-control assessment can include weight/volume, pH, specific gravity, active drug assay, color, rheologic properties/pourability, physical observation, physical stability (discoloration, foreign materials, gas formation, mold growth), and preservative-effectiveness test.2,3
Discussion: This preparation provides a sulfite-free formulation with variable concentrations for different patients.
5-ASA (mesalamine, Rowasa, Asacol, Canasa, Pentasa, C7H7NO3, MW 153.14) occurs as light tan to pink-colored, needle-shaped crystals. The color may deepen upon exposure to air. 5-ASA is odorless or may have a slight characteristic odor. 5-ASA is soluble in dilute hydrochloric acid and dilute alkali hydroxides, and it is slightly soluble in water.1
Ascorbic acid (L-ascorbic acid, vitamin C, C6H8O6, MW 176.12) occurs as white or slightly yellow crystals or an odorless powder. When exposed to light, it will gradually darken. Ascorbic acid is reasonably stable in air when dry, but in solution it rapidly oxidizes. It is freely soluble in water (1:3) and sparingly soluble in alcohol (1:40). Ascorbic acid should be stored in airtight, nonmetallic containers and protected from light. A 5% aqueous solution has a pH in the range of 2.1 to 2.6. Ascorbic acid solutions deteriorate rapidly in air.4
Methylcellulose (Methocel) is a practically odorless and tasteless, white to yellowish-white granule or powder that is widely used in both oral and topical formulations. It is available in different viscosity grades; the low viscosity grades are used to emulsify oils and as suspending and thickening agents for oral liquids, and the higher viscosity grades are used to thicken topically applied products such as creams and gels. The pH of a 1% solution is in the range of 5.5 to 8. Methylcellulose is practically insoluble in acetone, ethanol, and hot water; in cold water, it swells and disperses to form a viscous, colloidal dispersion. Its solutions are stable between pH values of 3 and 11, and the viscosity is decreased outside this pH range.5
Xanthan gum (corn sugar gum) is a high-molecular-weight polysaccharide gum containing, in each repeating unit, five sugar residues (two of d-glucose, two of D-mannose, and one of D-glucuronic acid). It has a molecular weight of approximately 2 × 106. Xanthan gum occurs as a cream-colored or white-colored, odorless, free-flowing, fine powder. It is soluble in cold or warm water but is practically insoluble in ethanol and ether. Xanthan gum is used as a stabilizing agent and as a viscosity-increasing agent in suspensions. It is nontoxic, is compatible with most other excipients, and has good stability (in the presence of enzymes, salts, acids, and bases) and viscosity properties over a wide range (pH 3-12). Xanthan gum solutions can tolerate up to about 60% water-miscible organic solvents, such as ethanol. The pH of a 1% w/v aqueous solution is in the range of 6 to 8.6
REFERENCES
1. U.S. Pharmacopeia/National Formulary [current revision]. Rockville, MD: U.S. Pharmacopeial Convention, Inc; November 2021.
2. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 1: physical and chemical testing. IJPC. 2019;23(3):211-216.
3. Allen LV Jr. Summary of quality-control testing for sterile and nonsterile compounded preparations, part 2: microbiological testing. IJPC. 2019;23(4):299-303.
4. Kibbe AH. Ascorbic acid. In: Sheskey PJ, Hancock BC, Moss GP, Goldfarb DJ, eds. Handbook of Pharmaceutical Excipients. 9th ed. London, England: Pharmaceutical Press; 2020:116-119.
5. Allen LV Jr. Methylcellulose. In: Sheskey PJ, Hancock BC, Moss GP, Goldfarb DJ, eds. Handbook of Pharmaceutical Excipients. 9th ed. London, England: Pharmaceutical Press; 2020:665-669.
6. Shah HC, Singh KK. Xanthan gum. In: Sheskey PJ, Hancock BC, Moss GP, Goldfarb DJ eds. Handbook of Pharmaceutical Excipients. 9th ed. London, England: Pharmaceutical Press; 2020:1129-1133.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





