US Pharm. 2014;39(12):HS18-HS23.
ABSTRACT: Liver cirrhosis is the result of chronic inflammation and fibrosis of the liver. This disease was recently found to be the 12th leading cause of death in the United States. Complications of cirrhosis include portal hypertension, varices, encephalopathy, ascites, and hepatorenal syndrome. Therapies directed at controlling these complications are targeted to reducing the hepatic-vein pressure gradient, reducing the risk of variceal bleeds, maintaining electrolyte balance, and preventing infections. Pharmacists should be cognizant of recommended drug-dosing alterations for patients with cirrhosis that are necessitated by decreased hepatic and/or renal function.
Liver cirrhosis is the end result of several mechanisms of liver inflammation caused by chronic liver disease, genetic disease, or autoimmune inflammation. Regardless of the inciting cause, liver cirrhosis follows a common pathologic process. The initial inflammatory response damages the hepatocytes, leading to necrosis and fibrosis of parenchymal tissue. Continual liver fibrosis and loss of functional hepatocytes lead to decreased functionality of the liver, which culminates in liver cirrhosis.1
Epidemiology
Cirrhosis continues to be a common cause of morbidity and mortality, and in 2011 it was the 12th leading cause of death in the United States. The main causes of cirrhosis are hepatitis B and C viruses, overuse of alcohol, and nonalcoholic liver disease. The exact prevalence of cirrhosis is unknown, owing to symptom development later in the disease process.2 The CDC reports that, in 2011, 10.8 deaths out of every 100,000 in the U.S. were attributed to chronic liver disease and cirrhosis.3
Management of Complications
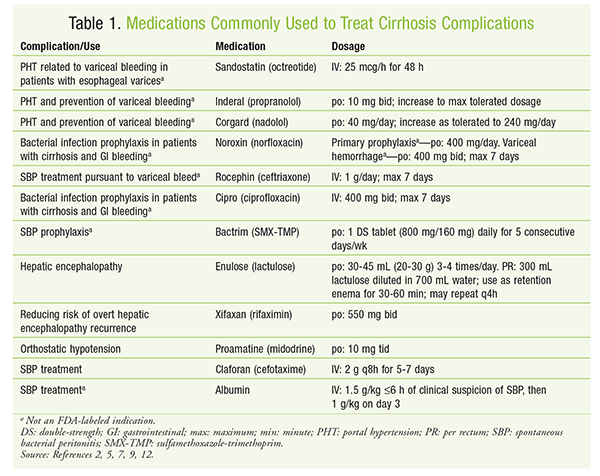
See TABLE 1 for a summary of medications commonly used to treat complications of cirrhosis.
Portal Hypertension: The majority of complications and deaths associated with cirrhosis are caused by portal hypertension. Portal hypertension is defined as a hepatic-vein pressure gradient (HVPG) of ≥5 mmHg. When the HVPG is <10 mmHg, patients have only a 10% probability of decompensating within the next 4 years. Once the HVPG increases to >10 mmHg, there is a significant rise in the development of esophageal varices, hepatocellular carcinoma, and overall mortality.2
Control of a patient’s portal hypertension may be accomplished through the use of nonselective beta-blockers such as propranolol and carvedilol. Although not indicated by the FDA, nonselective beta-blockers are helpful in reducing HVPG. The mainstay of drug therapy occurs in primary prophylaxis for preventing the bleeding of varices that have already developed. Both nonselective beta-blockers and endoscopic band ligation have proven effective in reducing bleeding and mortality in patients with existing varices. Nonselective beta-blockers reduce cardiac output and can reduce portal inflow through splanchnic vasoconstriction. Carvedilol, in particular, can be useful owing to its additional alpha-blocking properties, which help decrease intrahepatic vascular resistance. The dosage of a beta-blocker should be increased to the maximum tolerated.2,4
Acute Variceal Bleeding: Once a patient develops acute variceal bleeding, IV drug therapy is required for management. Treatment of an acute bleed should include vasoactive drugs, endoscopic band ligation, and antibiotics.2 Vasoactive drugs such as vasopressin and octreotide help reduce portal pressure by inhibiting the hormones responsible for vasodilation.5 This treatment should be administered for 2 to 5 days. Blood transfusions should be used to manage anemias that are due to bleeding. A target hemoglobin of 7 to 9 g/dL is appropriate.2,6 Transjugular intrahepatic portal-systemic shunts may also be required, especially in patients with Child-Pugh class B or C hepatic function.2
Infection: Broad-spectrum antibiotics are often required to control infections, as there is about a 20% occurrence of infection within 48 hours of a variceal bleed. The use of antibiotics helps prevent the occurrence of spontaneous bacterial peritonitis (SBP) and spontaneous bacteremia.7 SBP is a complication of infection of the ascitic fluid in the peritoneum. Diagnosis of SBP is made with an ascitic polymorphonuclear leukocyte count >250 cells/mm3 without another source of infection. The most commonly isolated pathogens are Enterobacteriaceae and Streptococcus and Enterococcus species.7,8 Based on the type of bacteria that are typically isolated, fluoroquinolones—especially norfloxacin and ciprofloxacin—have historically been recommended.7
In recent years, microbial resistance has been increasing, especially in healthcare settings. In patients with community-acquired infections and those without risk factors for multidrug-resistant organisms, a third-generation cephalosporin, such as cefotaxime, may be utilized.9 For more severe infections, including suspected multidrug-resistant and hospital-acquired infections, piperacillin-tazobactam or a carbapenem in addition to a glycopeptide such as vancomycin should be used.8 Antibiotics should be continued for 5 to 7 days because they result in a significant reduction in rebleeding and mortality in this patient population.2,6
Secondary prophylaxis is indicated in patients previously treated for SBP infection. Sulfamethoxazole-trimethoprim is the agent of choice in this situation, although norfloxacin also may be used. There is growing concern about bacterial resistance in patients with intermittent dosing, and daily dosing of prophylactic regimens may be warranted.9 See TABLE 1.
Encephalopathy: The development of encephalopathy in patients with cirrhosis is caused by an elevation in serum ammonia levels that is due to the loss of functional metabolism by the liver, and the condition is associated with significant mortality. Up to 64% of patients with encephalopathy will die within 1 year.2,10 Also, even minimal hepatic encephalopathy is associated with a significant risk of falls and driving impairment. Lactulose is the drug of choice for the prevention of recurrent encephalopathy.2 Oral lactulose may be effective in patients who are able to take medications by mouth. Rectal lactulose may be used in patients unable to tolerate oral dosing.5 If encephalopathy recurs despite appropriate treatment with lactulose, rifaximin may be added to further reduce the risk of recurrence.2,11,12 See TABLE 1.
International Normalized Ratio (INR) Elevation: One phenomenon that commonly occurs in patients with advanced liver cirrhosis is elevation of the INR value independent of pharmacologic intervention, mainly through the mechanism of decreased clotting-factor production. Importantly, this value is not associated with therapeutic anticoagulation. In fact, elevation of the INR value may represent a hypercoagulable state. Anticoagulation with unfractionated heparin should be used for prophylaxis of deep venous thrombosis in appropriate nonambulatory patients without a bleeding risk.13
Ascites: Ascites develops from a variety of mechanisms, including activation of the renin-angiotensin system, decreased oncotic pressure due to a low albumin level, and accumulation of fluid in the peritoneal space caused by the backflow of blood from the cirrhotic liver. Removal of the accumulated fluid, sometimes several liters in volume, may be accomplished by paracentesis. It is important to note that treatment of ascites caused by factors other than portal hypertension should be treated by targeting the underlying disorder. Patients with cirrhosis who develop ascites must first attempt steps such as alcohol cessation and diet modification. The combination of sodium reduction (not fluids in general) to <2,000 mg per day and diuretic therapy may also improve symptoms of ascites.9
Diuretics remain a cornerstone of fluid management in ascites, with treatment consisting mainly of furosemide and spironolactone in a 4-to-10 ratio starting at furosemide 40 mg and spironolactone 100 mg daily titrated every 3 to 5 days. The 4-to-10 ratio helps maintain normokalemia, with a maximum dosage of furosemide 160 mg and spironolactone 400 mg daily. ACE inhibitors, angiotensin receptor blockers, and nonsteroidal anti-inflammatory drugs (NSAIDs) should be used with caution or avoided.9
Refractory ascites is ascites that does not respond to sodium restriction and maximum-dose diuretic therapy or recurs despite recent paracentesis. Medication considerations in patients with refractory ascites include discontinuation of beta-blockers because of a possible increase in mortality and the addition of midodrine for orthostatic hypotension. In patients with a removal of >4 L of fluid by paracentesis, it is recommended to add albumin at a dosage of 6 to 8 g/L of fluid removed.9
Renal Complications
Liver cirrhosis can cause or exacerbate renal failure. This is due to various factors largely related to the decreased vascular resistance caused by complications of portal hypertension. In normal physiology, a decrease in vascular resistance is compensated for by an increase in cardiac output. Other methods of compensation involve the renin-angiotensin system, sympathetic system stimulation, and vasopressin release. The increased reuptake of sodium and water during these compensatory reactions worsens the ascites and edema commonly found in advanced cirrhosis. Vascular congestion and subsequent renal-artery hypoperfusion lead to renal failure. Other causes of hypovolemia and renal insufficiency include inflammation from infection and variceal bleeding.14
Hepatorenal Syndrome (HRS): HRS may present when serum creatinine is >1.5 mg/dL and is unresponsive to albumin administration after 48 hours of holding diuretics. The management of HRS in cirrhotic patients depends upon the inciting event. Other renal complications brought about by pharmacotherapy include hypovolemia-induced renal failure due to diarrhea-related excess fluid loss caused by lactulose administration, and drug-induced renal failure caused by NSAIDs or other nephrotoxic agents.14
Fluid administration may promote fluid retention, ascites, and hyponatremia, so this method should be avoided or used with caution. Nephrotoxic agents and blood products should be discontinued, and procedural measures should be instituted for bleeding.14
Hyponatremia treatment with vasopressin receptor antagonists (VRAs) such as tolvaptan have been shown to increase sodium concentrations in patients, including cirrhosis patients diagnosed with euvolemic and hypervolemic hyponatremia. It is important to note that the increase in sodium concentration has not been shown to improve clinical outcomes. The use of VRAs in this patient population is complicated by unpredictable and rapid correction of sodium levels, which places the patient at risk for demyelination.9
Drug-Dosing Considerations
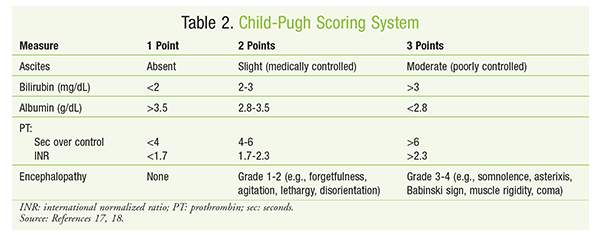
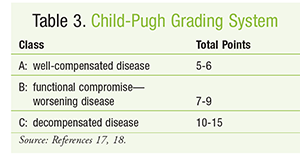
To determine relative disease severity and survival likelihood for patients expected to receive liver transplants, the model for end-stage liver disease (MELD) score can be calculated.15,16 The MELD score (FIGURE 1) is relatively consistent in predicting 3-month survival in patients with cirrhosis and also may be helpful in predicting prognosis after episodes of acute variceal bleeding. Increases in MELD score are associated with an increased risk of mortality.17 The Child-Pugh score (TABLES 2 and 3) is another method for estimating hepatic function in patients with alcoholic cirrhosis and portal hypertension.18 Dosage adjustments based on Child-Pugh score are described in the package inserts of numerous drugs. Common examples include ondansetron, venlafaxine, and voriconazole.19 It must be remembered that the Child-Pugh score does not have the sensitivity to quantify hepatic drug elimination in the manner that creatinine clearance calculation describes renal drug clearance.19

Conclusion
Cirrhosis is a condition that, over time, results in significant mortality. Complications of cirrhosis may require diverse pharmacotherapy. Given the effects of cirrhosis on the hepatic and/or renal drug metabolism pathways, pharmacists need to be aware of potential required dosage adjustments in this patient population.
REFERENCES
1. Zhou WC, Zhang QB, Qiao L. Pathogenesis of liver cirrhosis. World J Gastroenterol. 2014;20:7312-7324.
2. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761.
3. Hoyert DL, Xu J. Deaths: preliminary data for 2011. Nat Vit Stat Rep. 2012;61:6.
4. Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD; Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086-2102.
5. Clinical Pharmacology [database online]. Tampa, FL: Gold Standard, Inc; 2014. www.clinicalpharmacology.com. Accessed August 29, 2014.
6. Kim YD. Management of acute variceal bleeding. Clin Endosc. 2014;47:308-314.
7. Lee YY, Tee HP, Mahadeva S. Role of prophylactic antibiotics in cirrhotic patients with variceal bleeding. World J Gastroenterol. 2014;20:1790-1796.
8. Acevedo J, Fernández J. New determinants of prognosis in bacterial infections in cirrhosis. World J Gastroenterol. 2014;20:7252-7259.
9. Runyon BA; AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651-1653.
10. Jepsen P, Ott P, Andersen PK, et al. Clinical course of alcoholic liver cirrhosis: a Danish population-based cohort study. Hepatology. 2010;51:1675-1682.
11. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735.
12. Xifaxan (rifaximin) product information. Raleigh, NC: Salix Pharmaceuticals, Inc; March 2014.
13. Koliscak L, Maynor L. Pharmacologic prophylaxis against venous thromboembolism in hospitalized patients with cirrhosis and associated coagulopathies. Am J Health Syst Pharm. 2012;69:658-663.
14. Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279-1290.
15. Mauss S, Berg T, Rockstroh J, et al. Hepatology: A Clinical Textbook. 5th ed. Flying Publisher; 2014. www.liver.ca/files/Professional_Education___Partnerships/Information___Resources_for_HCP/Hepatology_2014_-_A_Clinical_Textbook.pdf. Accessed November 5, 2014.
16. DiPiro JT, Talbert RL, Yee GC, et al. Pharmacotherapy: A Pathophysiologic Approach. 9th ed. New York, NY: McGraw-Hill Education; 2014.
17. Thayalasekaran S, Tsochatzis EA. Is MELD the best prognostic score in acute variceal bleeding? The jury is still out. Ann Gastroenterol. 2014;27:282-283.
18. Verbeeck RK. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol. 2008;64:1147-1161.
19. Spray JW, Willett K, Chase D, et al. Dosage adjustment for hepatic dysfunction based on Child-Pugh scores. Am J Health Syst Pharm. 2007;64:690,692-693.
To comment on this article, contact rdavidson@uspharmacist.com.





