US Pharm. 2019;44(6)(Generic Drugs suppl):36-39.
ABSTRACT: With the entry of biosimilars into the U.S. market, it is important for pharmacists to understand these products and how they differ from generic medications. Unlike generics, biosimilars are not identical to the reference biological product. Because biological products are made using living cells and processes, they may have minor differences from the reference product. For approval, biosimilars must demonstrate that these differences are not clinically meaningful. Nonetheless, certain factors should be considered when a switch from the reference product to a biosimilar is contemplated. For this reason, pharmacists have an opportunity to educate patients and providers to ensure appropriate use.
Biological products, which are FDA-regulated, are large, complex proteins that are used in the treatment of diseases such as various cancers, rheumatoid arthritis, and diabetes.1-3 Unlike traditional small-molecule medications, which have standard production methods and well-defined structures, biological products have a sophisticated manufacturing process that involves the use of cell cultures. This process can result in heterogeneous products with slight variations in manufacturing.1,4-7 Biological products have transformed therapy in several fields; however, their prohibitive prices, which stem from the costs of research and development and manufacturing, are a concern.8-10 Fortunately, the Biologics Price Competition and Innovation Act (BPCIA), which was signed into law as part of the Patient Protection and Affordable Care Act of 2010, has created a pathway for the approval of biosimilars that has helped make biological products more affordable.9
Biosimilar Products
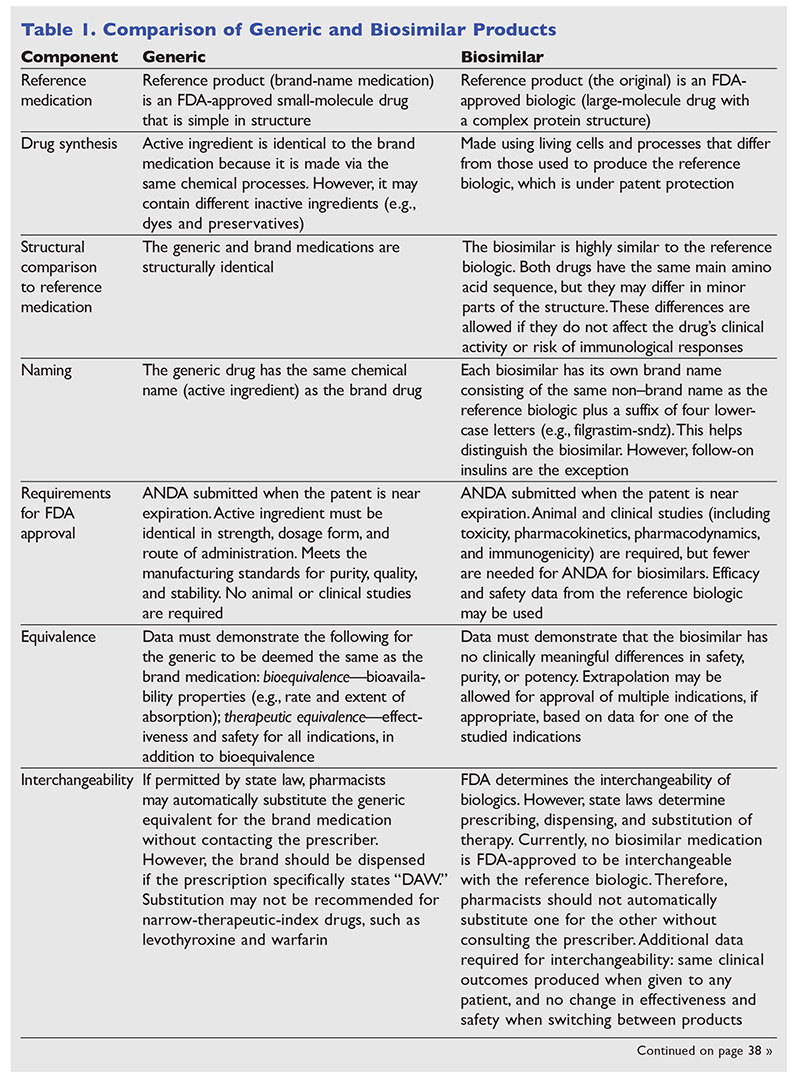
Biosimilar products are biological products that are manufactured to resemble reference biological products.5,7 The BPCIA permits the FDA, following a period of market exclusivity for reference products, to approve biological products if they have the same primary amino acid sequence and mechanism of action as the reference product and there are no clinically meaningful differences between the reference product and the biosimilar.3,4,8,11 Because each reference product's manufacturing process is proprietary information, the manufacturer's biosimilar product always differs slightly from the reference product.4,7 This is in contrast to generic medications, which are identical to brand medications.8 Therefore, although there are some similarities between generic and biosimilar medications, biosimilars are not considered generic versions of biological products. See TABLE 1 for a comparison of biosimilars and generic medications.4-8,11-18
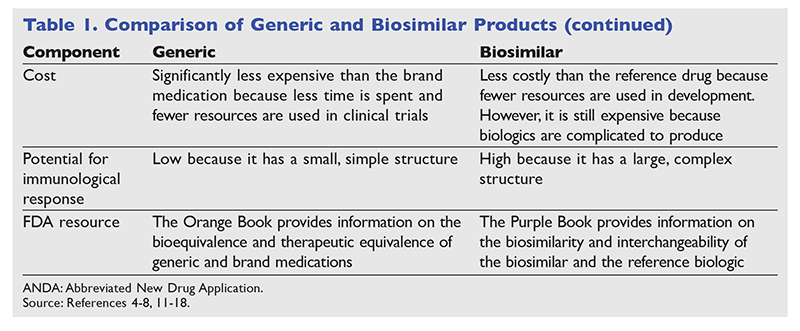
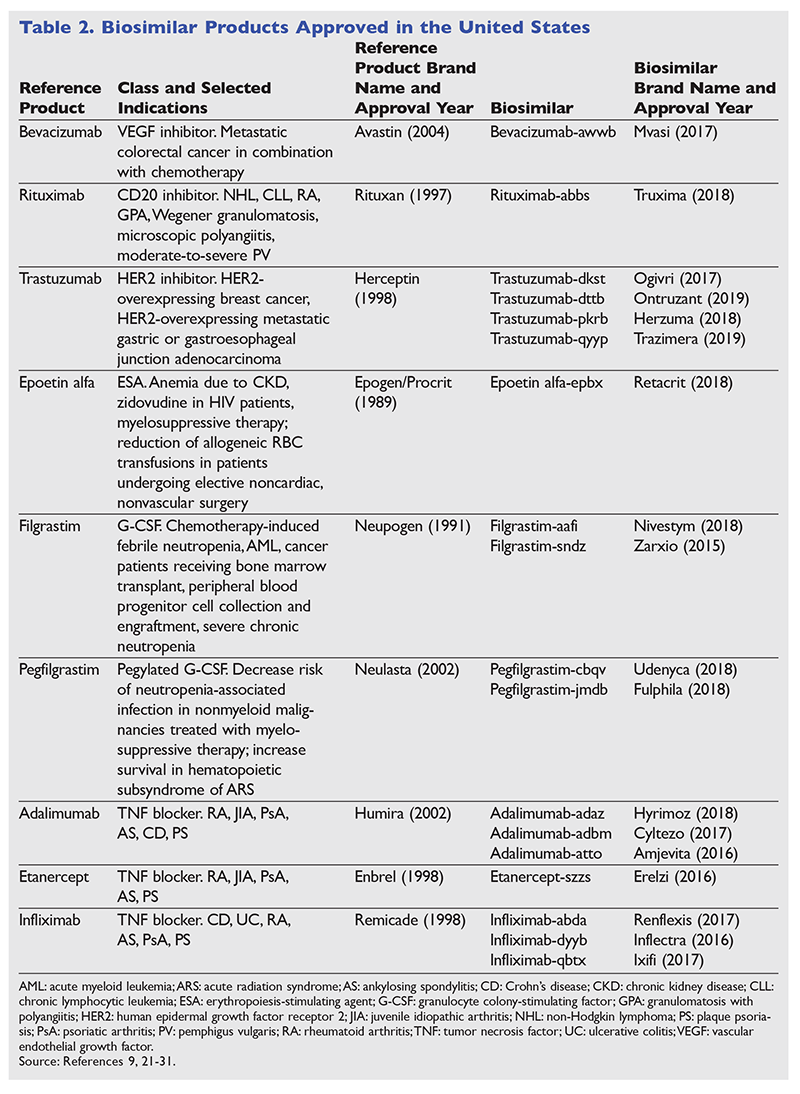
Biological products have enabled significant advances in the treatment of cancer, cancer-related complications, and rheumatologic and musculoskeletal diseases.8,19 Patent expiration of reference biological products has stimulated the development of multiple biosimilar products in recent years.20 The ongoing approval of biosimilars allows for the potential of cost containment and affordability.8,9 As of March 2019, there were nine biological products with FDA-approved biosimilars (TABLE 2).8,9,21-31
There is a theoretical increase in immunological response when a patient is switched from one biological product to another, be it a reference product or a biosimilar.26 Immunological responses include anaphylaxis, infusion reactions, neutralizing antibodies, and reduced efficacy.5,6,26 These reactions may occur months after initiation, making it important for clinicians to monitor and report suspected immunological responses.6 Because these are newer medications, postmarketing surveillance is also essential for identifying adverse events from biosimilars.7
Follow-on Insulins
Follow-on insulins are designated as follow-on biologics rather than biosimilars because they undergo an older approval pathway. Abbreviated New Drug Applications for follow-on biologics are submitted through Section 505(b)(2) of the Food, Drug, and Cosmetic Act. After March 23, 2020, all biologics will be regulated through Section 351(k) under the Public Health Service Act and will be referred to as biosimilars.13 TABLE 3 lists the current follow-on insulins.32,33
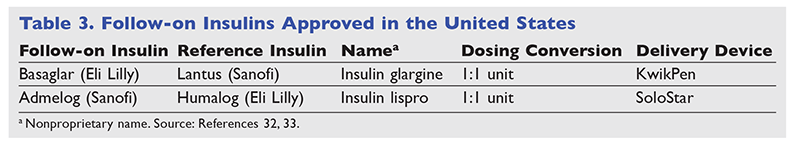
Basaglar is the first follow-on insulin to be approved in the United States. The baseline reduction in A1C between Basaglar and Lantus in the ELEMENT I and II noninferiority studies was nonsignificant.34,35 The nonproprietary name is used without the additional suffix, so it is important to check that prescriptions for insulin glargine are specified as either Basaglar or Lantus. Because of Basaglar's lower price, many insurance companies have replaced Lantus with Basaglar on their formularies. Although the dosing remains the same for the follow-on insulin, the delivery device (e.g., SoloStar vs. KwikPen) may differ between companies. This is permitted by the FDA as long as there is demonstrable compatibility with the device. Patients should be educated on the changes associated with delivery devices because ease and comfort may differ from what they are used to.13
In March 2019, Eli Lilly announced the release of a generic version of its own brand insulin, Humalog, at half the price ($265.20 for five pens).36 Prior to this, Sanofi's Admelog became available as Humalog's follow-on insulin; it is also less expensive than the brand drug. Even with these options, the cost of insulin is still unaffordable for many patients and illustrates the need for more affordable options.
Conclusion
The lower price of biosimilars and follow-on biologics makes them appealing alternatives to branded biologics. These cost savings may drive insurance companies and institutions to switch to biosimilars in their formularies. Therefore, it is crucial for clinicians to be aware of how switching from a reference biologic to a biosimilar differs from switching from a brand to a generic medication. Because biosimilars are not interchangeable, the available data must be evaluated to determine the appropriateness of the switch and to ensure that effectiveness and safety are not compromised. Pharmacists should be aware that biosimilars may not be substituted without prescriber involvement. The introduction of interchangeable biosimilars may occur in the future and could allow for substitution, if permitted by state law. As the use of biosimilars increases, pharmacists will serve an increasingly vital role in educating prescribers and patients regarding the particulars of switching from reference biologics to biosimilars. Pharmacists can also clarify misconceptions surrounding safety and effectiveness that may deter the use of biosimilars. Any changes in medication delivery device resulting from the switch should be explained to the patient. Finally, because biosimilars have been on the market for only a few years, it is important for pharmacists to recognize and report adverse events.
REFERENCES
1. Pagani E. Why are biosimilars much more complex than generics? Einstein (São Paulo). 2019;17(1):eED4836.
2. Hung A, Vu Q, Mostovoy L. A systematic review of U.S. biosimilar approvals: what evidence does the FDA require and how are manufacturers responding? J Manag Care Spec Pharm. 2017;23(12):1234-1244.
3. Biosimilars Action Plan: Balancing Innovation and Competition. Silver Spring, MD: FDA; 2018.
4. Stevenson JG, Popovian R, Jacobs I, et al. Biosimilars: practical considerations for pharmacists. Ann Pharmacother. 2017;51(7):590-602.
5. Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health Syst Pharm. 2013;70(22):2004-2017.
6. Griffith N, McBride A, Stevenson JG, Green L. Formulary selection criteria for biosimilars: considerations for US health-system pharmacists. Hosp Pharm. 2014;49(9):813-825.
7. Li E, Ramanan S, Green L. Pharmacist substitution of biological products: issues and considerations. J Manag Care Spec Pharm. 2015;21(7):532-539.
8. Lyman GH, Balaban E, Diaz M, et al. American Society of Clinical Oncology statement: biosimilars in oncology. J Clin Oncol. 2018;36(12):1260-1265.
9. Campen CJ. Integrating biosimilars into oncology practice: implications for the advanced practitioner. J Adv Pract Oncol. 2017;8(7):688-699.
10. Boyle RM. The use of biologics in cancer therapy. US Pharm. 2010;35(3)(Oncology suppl):4-7.
11. FDA. Biosimilar and interchangeable products. www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicapplications/Biosimilars/ucm580419.htm#generic. Accessed March 21, 2019.
12. FDA. Generic drugs: questions & answers. www.fda.gov/Drugs/ResourcesForYou/Consumers/QuestionsAnswers/ucm100100.htm. Accessed March 25, 2019.
13. Dolinar R, Lavernia F, Edelman S. A guide to follow-on biologics and biosimilars with a focus on insulin. Endocr Pract. 2018;24(2):195-204.
14. FDA. Biosimilar development, review, and approval. www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/TherapeuticBiologicApplications/Biosimilars/ucm580429.htm. Accessed March 21, 2019.
15. FDA. Approved drug products with therapeutic equivalence evaluations, 39th ed. www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/UCM071436.pdf. Accessed May 10, 2019.
16. Vivian JC. Generic-substitution laws. US Pharm. 2008;33(6)(Generic Drug Review):30-34.
17. Pope ND. Generic substitution of narrow therapeutic index drugs. US Pharm. 2019;34(6)(Generic Drug Review):12-19.
18. FDA. Purple Book: lists of licensed biological products with reference product exclusivity and biosimilarity or interchangeability evaluations. www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/therapeuticbiologicapplications/biosimilars/ucm411418.htm. Accessed March 25, 2019.
19. Mysler E, Pineda C, Horiuchi T, et al. Clinical and regulatory perspectives on biosimilar therapies and intended copies of biologics in rheumatology. Rheumatol Int. 2016;36(5):613-625.
20. Declerck P, Danesi R, Petersel D, Jacobs I. The language of biosimilars: clarification, definitions, and regulatory aspects. Drugs. 2017;77(6):671-677.
21. Center for Biologic Evaluation and Research. CBER list of licensed biological products. www.fda.gov/media/89426/download. Accessed May 10, 2019.
22. Avastin (bevacizumab) package insert. South San Francisco, CA: Genentech, Inc; February 2019.
23. Rituxan (rituximab) package insert. South San Francisco, CA: Genentech, Inc; 1997.
24. Herceptin (trastuzumab) package insert. South San Francisco, CA: Genentech, Inc; 1998.
25. Epogen (epoetin alfa) package insert. Thousand Oaks, CA: Amgen, Inc; 1989.
26. Rifkin RM, Peck SR. Biosimilars: implications for clinical practice. J Oncol Pract. 2017;13(suppl 9):S24-S31.
27. Neupogen (filgrastim) package insert. Thousand Oaks, CA: Amgen, Inc; 1991.
28. Neulasta (pegfilgrastim) package insert. Thousand Oaks, CA: Amgen, Inc; 2002.
29. Humira (adalimumab) package insert. North Chicago, IL: Abbott Laboratories; 2002.
30. Enbrel (etanercept) package insert. Thousand Oaks, CA: Amgen, Inc; 1998.
31. Remicade (infliximab) package insert. Horsham, PA: Janssen Biotech, Inc; 1998.
32. Basaglar (insulin glargine injection) package insert. Indianapolis, IN: Eli Lilly and Co; September 2018.
33. Admelog (insulin lispro injection) package insert. Bridgewater, NJ: Sanofi-Aventis U.S. LLC; December 2017.
34. Blevins TC, Dahl D, Rosenstock J, et al. Efficacy and safety of LY2963016 insulin glargine compared with insulin glargine (Lantus®) in patients with type 1 diabetes in a randomized controlled trial: the ELEMENT 1 study. Diabetes Obes Metab. 2015;17(8):726-733.
35. Hadjiyianni I, Dahl D, Lacaya LB, et al. Efficacy and safety of LY2963016 insulin glargine in patients with type 1 and type 2 diabetes previously treated with insulin glargine. Diabetes Obes Metab. 2016;18(4):425-429.
36. Eli Lilly and Co. Lilly to introduce lower-priced insulin. www.prnewswire.com/news-releases/lilly-to-introduce-lower-priced-insulin-300805560.html. Accessed March 25, 2019.
To comment on this article, contact rdavidson@uspharmacist.com.
« Click here to return to Generic Drug Update.






