US Pharm. 2021;46(12):22-30.
ABSTRACT: The annual influenza vaccine is the best way to reduce the risk of seasonal influenza illnesses. However, due to the limitations of the influenza vaccine and influenza-related complications, antiviral medications were developed to overcome these challenges and serve as an additional line of defense. There are four FDA-approved antiviral drugs recommended by the CDC to treat influenza, two of which are available orally: oseltamivir phosphate and baloxavir marboxil. Differences exist in their uses, dosing, and how they work. Appropriate use of these agents in both treatment and prophylaxis should be carefully considered.
In a post-COVID world, seasonal influenza has been effectively dethroned. Masking mandates, increased vaccination rates, and quarantines might have diluted the global effect of the influenza virus; however, it still poses a significant threat to public health.1-4 The most effective treatment of the virus is prevention through annual vaccination, although that is not without caveats.3,5 The influenza vaccine is majorly limited by the virus’ constant mutation, requiring annual reformulations and what are essentially “best guesses” with regard to targeted strains. Development of the vaccine and medications are significantly handicapped by both the nature of the virus and increasing resistance rates, leading to a call for new drug therapies as well.1,3,4,6 Maintenance of influenza surveillance has also suffered during the pandemic, leading to less evidence characterizing the influenza virus and, consequently, limitations in the development of the 2021-22 flu vaccine.2 While focus and funding of research have understandably been targeted toward COVID-19 in recent months, it is imperative that healthcare professionals remain vigilant toward the virus that once held much more attention. Considering the limitations of the flu vaccine and the imminence of the current flu season, a review of treatment options for seasonal influenza is vital; and increasing resistance rates call for increased stewardship of our currently available therapies. This review article will focus on the oral influenza therapies commonly prescribed in the community.
Overview of Oral Influenza Therapies
Antiviral therapies for influenza were developed to shorten the disease duration, improve recovery, and reduce the risk of influenza-associated complications. Currently, three antiviral classes of medications exist in the treatment of influenza: adamantane antivirals (M2 inhibitors), neuraminidase inhibitors (NAIs), and nucleoprotein inhibitors.1 Adamantane antivirals (amantadine and rimantadine) fell out of favor almost 2 decades ago due to increased resistance, limitation to influenza A viruses, and poor tolerability; thus, they are no longer recommended.
Three NAIs are currently approved for and used in the treatment of influenza in the United States, including zanamivir (Relenza), oseltamivir (Tamiflu), and peramivir (Rapivab). NAIs are the only drug class recommended in the 2018 Infectious Diseases Society of America (IDSA) clinical practice guidelines.7 NAIs work by inhibiting neuraminidase, the enzyme that cleaves glycosidic bond of sialic acid, which ultimately allows the new virion to be released from the host cell and consequently infect other cells.1 Oseltamivir is the only NAI with sufficient oral bioavailability to be formulated as capsules and liquid, and it targets both influenza strains A and B. The single nucleoprotein inhibitor baloxavir (Xofluza) is an oral anti-influenza agent that was approved by the FDA in 2018, and thus it is not included in the most current IDSA guidelines. This cap-dependent endonuclease inhibitor works by inhibiting transcription of viral mRNA through targeting the viral polymerase, thus inhibiting viral replication.1,8 By targeting different parts of the viral life span, release and replication, these two agents can be uniquely efficacious.
Oseltamivir phosphate is a phosphate prodrug of oseltamivir, which is rapidly absorbed and extensively metabolized to oseltamivir carboxylate (OC) by esterases found throughout the body.1 With this rapid absorption, detectable levels of OC are present in plasma within 30 minutes of dosing, and time to maximum concentration (Tmax) is reached after 3 to 4 hours.9 Rapid absorption is a very desirable trait when discussing oseltamivir, as it is most effective when administered within 48 hours of onset of symptoms. Without this phosphate salt formulation, oseltamivir has low lipophilicity and oral bioavailability, which would render it ineffective as an oral treatment option.
Oseltamivir and OC are well distributed throughout the body and, therefore, are able to reach therapeutic levels in the lungs, sinuses, and nasal mucosa, making it an ideal agent for a susceptible respiratory virus.9 As mentioned, oseltamivir phosphate is extensively metabolized, around 75%, to OC by first-pass metabolism from hepatic esterases. Neither the phosphate prodrug nor OC is metabolized through CYP-mediated interactions, and therefore there are no CYP-mediated drug interactions associated with them. OC and oseltamivir phosphate are primarily cleared by renal elimination through both glomerular filtration and renal tubular secretion via anionic transport process.9
Baloxavir marboxil is a prodrug that is rapidly metabolized to baloxavir acid by hydrolysis.10 Baloxavir has a similar Tmax to oseltamivir, roughly 3.5 hours.10 In contrast to oseltamivir, baloxavir demonstrated a half-life of 49 to 91 hours, which is favorable for dosing regimen as baloxavir is administered as a single-dose regimen with no follow-up doses required.10 Fed-state or before-meals administration of baloxavir were found to have a significant impact on drug concentrations, as they resulted in a decreased AUC of 37% to 47% compared with administration in the fasted state.10
Safety and Efficacy
When considering an oral flu regimen, it is also important to consider the safety data, particularly in patients with underlying diseases, younger children and infants, elderly patients, and pregnant patients, as these special populations are still at risk of complications associated with influenza. While oseltamivir has plenty of data supporting its safety in younger populations and pregnancy, one study targeted further assessment of these safety findings at standard and higher doses of oseltamivir in healthy adults aged 18 to 65 years. In this particular study, participants were given one of four treatment regimens (placebo, 75 mg twice daily, 225 mg twice daily, or 450 mg twice daily each for 5 days).11
The most frequently reported adverse events were headache, nausea/vomiting, dizziness, and hot flushes.11 Of these, headache was the most common; it was noted in about 17% to 24% of participants and did not show any relationship to increasing dosages of oseltamivir.11 Nausea and vomiting did appear to be dose-related, but most cases occurred on Day 1 and resolved within 24 hours.11 Hot flushes also appeared to be dose-related and occurred in a few patients from each of the three oseltamivir groups. Out of 391 participants, there were seven withdrawals, three of which were from the placebo group, and no withdrawals from the 450-mg bid group. Of the other four withdrawals, only two were due to adverse events, both of which were in the 225-mg bid group.11 As evidenced by this study, oseltamivir has been proven safe even at doses much higher than that currently indicated for influenza treatment or prophylaxis. As previously alluded to, oseltamivir has also been studied in various special populations with well-documented safety data.
Baloxavir is a newer influenza agent and, therefore, does not have the overwhelming safety data from trials compared with oseltamivir. Regardless, the results from the phase III clinical trial show baloxavir to have fewer adverse events compared with oseltamivir.12 Two serious adverse events did occur in the baloxavir treatment group, but neither were considered to be related to the trial regimen.12 The adverse events observed in the phase III clinical trial that were considered to be related to the trial regimen were diarrhea and nausea.12 Diarrhea occurred more often in the baloxavir group (1.8% vs. 1.4%), while nausea occurred more often in the oseltamivir group (0.3% vs. 1.6%).12 Given the results of this clinical trial, while there are not as much safety data for baloxavir as there are for oseltamivir, both treatment options have established safety data to support their use in current influenza infections.
While the safety of these drugs is established, the efficacy has generally been held under closer inspection. Viruses pose unique challenges in drug development—as evident in the paucity of antiviral therapy relative to other antimicrobials and especially in those specifically directed at influenza. Though the virus is responsible for over 30 million cases annually, there are only two available oral options, oseltamivir and baloxavir, which are quickly gaining resistance.13 NAIs are heralded as agents that can limit the severity and duration of influenza, which is essentially the reach of their efficacy. Oseltamivir has been well studied in multiple, specific populations, including pregnant women and adolescents. Notably, its effects are also time-dependent, where trials found that receipt of the drug within 48 hours of symptom onset greatly improved clinical outcomes. A meta-analysis estimated the reduction in time to first alleviation of symptoms to be approximately 16 to 18 hours upon administration of oseltamivir.14 These results are limited to uncomplicated disease, as efficacy of oseltamivir in severe cases of influenza is inconclusive, especially with regard to hospitalization and mortality. Additionally, resistance to agents such as oseltamivir has increased, best exhibited by the drug-resistant H274Y/H1N1 influenza virus, or “swine flu” strain responsible for the 2009 pandemic.3,4
Baloxavir is perhaps the newest widely used influenza agent, despite its absence from the IDSA guidelines. Its single-dose formula has quickly made it a desirable option in the treatment of influenza. Additionally, trials also found it to have better efficacy against the strains resistant to NAIs. In the CAPSTONE-1 phase III trial, patients older than age 12 years treated with baloxavir had a median time to alleviation of symptoms of ~54 hours compared with ~80 hours with placebo. In this trial, baloxavir had similar results to oseltamivir in time to alleviation of symptoms but did have greater reductions in viral load 1 day after administration compared with placebo and oseltamivir. Though baloxavir displayed similar clinical efficacy to oseltamivir in this trial with regard to influenza A, it did display superiority to oseltamivir when treating influenza B.8 These results were confirmed by the CAPSTONE-2 trial.15 Unfortunately, while no resistant strains had been identified at the time of approval, some resistance to baloxavir has emerged. It lacks evidence in special populations as well. Baloxavir, like oseltamivir, does not have evidence supporting use in complicated or severe cases. Monitoring of resistance, as well as research in these complicated populations, is warranted in order to fully understand the extent of efficacy oseltamivir and baloxavir have against influenza.4
Other Considerations
As with many medications, oseltamivir and baloxavir may cause a severe skin reaction and/or a serious allergic reaction, including anaphylaxis.16 In either case, the patient should be counseled to stop the medication immediately and seek medical attention right away. While rare, oseltamivir also carries the risk of neuropsychiatric events, and any patient experiencing abnormal behavior should be instructed to stop the medication and contact their physician.16 If a dose of oseltamivir is missed, the patient should be instructed to take the dose as soon as they remember, unless it is within 2 hours of their next scheduled dose.16 While baloxavir does not carry the same risks as oseltamivir, patients should be advised to avoid milk, calcium-rich beverages, antacids, or supplements around the time that they take their single dose of baloxavir.17
Place in Therapy
Rising resistance rates and a potential surge in demand for influenza medications this flu season warrants a closer look at their appropriate use and place in therapy. Oseltamivir and baloxavir are both commonly inappropriately prescribed.18 The unique timing criteria for both agents and general lack of options create a perfect storm for misuse. Baloxavir has not been studied in patients presenting after 48 hours of symptom onset, and oseltamivir studies have revealed a decline in efficacy after 48 hours of symptom onset, with no evidence suggesting any clinical utility thereafter.18,19 Guidelines recommend against using these medications after this time period unless the illness is severe and progressing in a hospitalized patient, which is supported only by observational studies.13 Inappropriate prescribing has been associated with a lack of knowledge of their appropriate use, the belief that they might have some efficacy after 48 hours, or the desire to ensure patient satisfaction by providing a prescription. Evidence suggests there is, in fact, a gap in provider knowledge, and increased education might be warranted.18
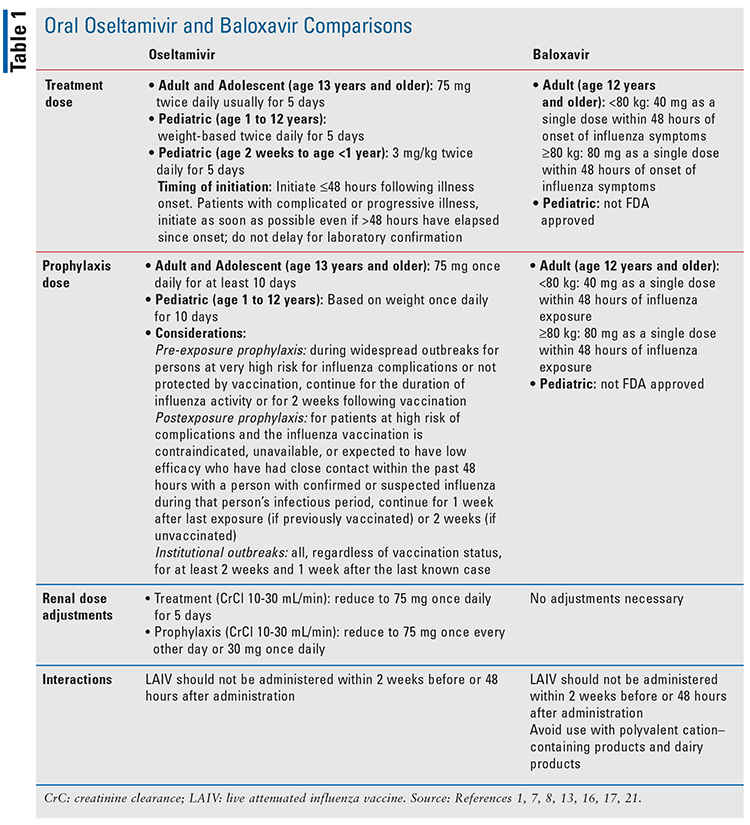
IDSA guidelines include only NAIs in their recommendations, of which oseltamivir is recommended as first line.7 However, the advantages found with baloxavir, as well as its presumed efficacy, have given it an established place in therapy. Baloxavir is not approved for use in chemoprophylaxis like oseltamivir, but rather only in treatment. Guidelines ultimately suggest, however, that the influenza vaccine is the primary recommended means of prevention.7 Recently, combination therapy using the two agents has been studied, and a benefit over monotherapy in mice was found, although more research is needed in to confirm this.20 Further details regarding appropriate use of oseltamivir and baloxavir can be found in TABLE 1.
Conclusion
A review of oral influenza therapy is necessary and timely given the expected severity of the current flu season, the questions surrounding the current flu vaccine, and rising resistance rates towards agents. Oseltamivir and baloxavir are the only two oral agents available, and they are most commonly seen in the community. Fortunately, their safety is well established, and other concerns such as adverse effects or drug interactions are relatively mild. Unfortunately, efficacy is not as curative as desired but rather reduces duration and symptom severity. Importantly, clinical utility is only seen when administered within 48 hours of symptom onset, and no evidence exists to suggest efficacy thereafter. Appropriate use of these agents in both treatment and prophylaxis should therefore be carefully considered. Education of providers might be warranted to preserve the already limited arsenal of influenza agents and to provide optimal care to patients in the community.
REFERENCES
1. Shie JJ, Fang JM. Development of effective anti-influenza drugs: congeners and conjugates—a review. J Biomed Sci. 2019;26:84.
2. Rubin R. Influenza’s unprecedented low profile during COVID-19 pandemic leaves experts wondering what this flu season has in store. JAMA. 2021;326(10):899-900.
3. Yin H, Jiang N, Shi W, et al. Development and effects of influenza antiviral drugs. Molecules. 2021;26:810.
4. Principi N, Camilloni B, Alunno A, et al. Drugs for influenza treatment: is there significant news? Front Med (Lausanne). 2019;6:109.
5. Gavin PJ, Thomson RB Jr. Review of rapid diagnostic tests for influenza. Clin Applied Immunol Rev. 2004;4:151-172.
6. Treanor J. Influenza challenge and the challenge of drug development. J Infect Dis. 2019;219:171-172.
7. Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the Infectious Diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenza. Clin Infec Dis. 2019;68:895-902.
8. Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379(10):913-923.
9. Davies BE. Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J Antimicrob Chemother. 2010;65(Suppl 2):ii5-ii10.
10. Koshimichi H, Ishibashi T, Kawaguchi N, et al. Safety, tolera¬bility, and pharmacokinetics of the novel anti-influenza agent bal¬oxavir marboxil in healthy adults: phase I study findings. Clin Drug Investig. 2018;38:1189-1196.
11. Dutkowski R, Thakrar B, Froehlich E, et al. Safety and phar¬macology of oseltamivir in clinical use. Drug Saf. 2003;26:787-801.
12. Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med. 2018;379:913-923.
13. CDC. Influenza antiviral medications: summary for clinicians. Updated September 22, 2021. https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm?web=1&wdLOR=c07FAF0A5-
A982-E640-8BFF-3987955EC0FA. Accessed September 25, 2021.
14. Dobson J, Whitley RJ, Pocock S, et al. Oseltamivir treatment for influenza in adults: a meta-analysis of randomised controlled trials [published correction appears in Lancet. 2015;385(9979):1728]. Lancet. 2015;385(9979):1729-1737.
15. Ison MG, Portsmouth S, Yoshida Y, et al. Early treatment with baloxavir marboxil in high-risk adolescent and adult outpatients with uncomplicated influenza (CAPSTONE-2): a randomised, placebo-controlled, phase 3 trial. Lancet Infect Dis. 2020;20(10):1204-1214.
16. Tamiflu (oseltamivir) [package insert]. South San Francisco, CA: Genentech, Inc. August 2019.
17. Xofluza (baloxavir marboxil) [package insert]. South San Francisco, CA: Genentech, Inc. March 2021.
18. Kazempoor D. Healthcare provider education on oseltamivir resistance and Centers for Disease Control and Prevention prescrip¬tion guidelines of oseltamivir for influenza. UCLA. ProQuest ID: Kazempoor_ucla_0031D_19742. Merritt ID: ark:/13030/m5wt4rp9. Retrieved from https://escholarship.org/uc/item/5hs3h7bh.
19. McLean HQ, Belongia EA, Kieke BA, et al. Impact of late oseltamivir treatment on influenza symptoms in the outpatient setting: results of a randomized trial. Open Forum Infect Dis. 2015;2:ofv100
20. Fukao K, Noshi T, Yamamoto A, et al. Combination treatment with the cap-dependent endonuclease inhibitor baloxavir marboxil and a neuraminidase inhibitor in a mouse model of influenza A virus infection. J Antimicrob Chemother. 2019;74(3):654-662.
21. Ikematsu H, Hayden FG, Kawaguchi K, et al. Baloxavir marboxil for prophylaxis against influenza in household contacts. N Engl J Med. 2020;383(4):309-320.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





