US Pharm. 2008;33(5)(Diabetes suppl):3-9.
Diabetes mellitus is a group of
metabolic disorders in which the body does not produce enough or properly use
insulin, resulting in hyperglycemia. Two major types of diabetes mellitus are
recognized. Type 1, previously known as juvenile diabetes, is the result of
the body's failure to produce enough insulin. This type of diabetes is
prevalent in 5% to 10% of the diabetic population and is generally diagnosed
in children.1 Type 2 diabetes is a result of insulin resistance (a
condition in which the body cannot properly use insulin). This type of
diabetes is more prevalent and is generally diagnosed in adults.
Incidences of diabetes are
increasing at epidemic proportions. According to the estimates of the World
Health Organization, there are more than 180 million people with diabetes
worldwide and this number is likely to more than double by 2030.2
The American Diabetes Association estimates that more than 20 million adults
and children in the United States (which accounts for approximately 7% of the
U.S. population) have diabetes.3 While more than 14 million
patients have been diagnosed with diabetes, over six million individuals are
estimated to be unaware of the fact that they have the disease and remain
untreated.3
Diabetes-Associated
Complications
Diabetes is
associated with a number of clinical complications such as cardiopathy,
nephropathy, and retinopathy. Many individuals are not even aware that they
have diabetes until they are diagnosed with one of these complications.
Unmanaged diabetes can lead to life-threatening complications such as heart
disease, stroke, blindness, and kidney failure.1 Diabetes is the
sixth leading cause of death in the U.S.
Hepatotoxicity in the
Diabetic Population
Until now, the link
between risk of hepatic failure and diabetic condition was unclear. Recently,
a large prospective cohort study was performed to examine whether patients
with type 2 diabetes are at an increased risk of developing acute liver
failure.5 This study suggested that diabetic patients are twice as
likely to suffer hepatic failure compared to normal patients. Another study of
the same prospective cohort population indicated that diabetes is associated
with increased risk of hepatocellular carcinoma and chronic liver diseases.
6
Hepatotoxicity did not receive
as much attention as other prevalent complications (i.e., cardiopathy,
retinopathy, nephropathy) until hepatotoxicity of antidiabetic drugs emerged
as a common clinical complication. This article is an attempt to summarize the
current understanding of hepatotoxicity of antidiabetic drugs.
Hypoglycemic Agents in the
Treatment of Diabetes
Many therapeutic
drugs target both fasting and postprandial hyperglycemia and other metabolic
parameters involved in the diabetes-associated complications. These drugs are
directed towards increasing insulin secretion, decreasing insulin resistance,
and increasing insulin penetration into the cells. Antidiabetic drugs with
reported cases of hepatotoxicity include sulfonylureas, alpha-glucosidase
inhibitors, biguanides, and thiazolidinediones (Table 1).
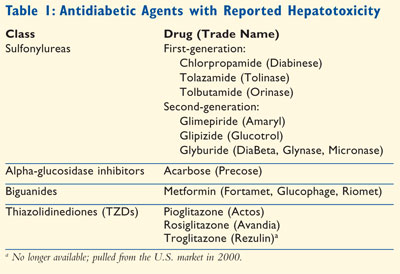
Sulfonylureas
Sulfonylureas have
been used as first-line oral antihyperglycemic agents for type 2 diabetes
since 1954. First-generation sulfonylureas include tolbutamide (Orinase),
tolazamide (Tolinase), and chlorpropamide (Diabinese). Chlorpropamide and
tolbutamide are well recognized as causes of hepatotoxicity.7
However, there have been only three reported cases of hepatic injury caused by
a third oral hypoglycemic agent, tolazamide.7 With the arrival of
second-generation sulfonylureas, first-generation sulfonylureas are rarely
used. Second-generation sulfonylureas include glipizide (Glucotrol), glyburide
(DiaBeta, Micronase, Glynase), and glimepiride (Amaryl). Drug-induced
hepatotoxicity has been reported infrequently with second-generation
sulfonylureas. For glimepiride, a second-generation sulfonylurea, there have
been no reports of hepatotoxicity in English literature; however,
hepatotoxicity has been reported in French literature.8,9
Alpha-Glucosidase Inhibitors
The glucosidase
inhibitors are useful adjunctive therapies for type 2 diabetes. The prototype
of this class is acarbose (Precose). Because acarbose is minimally absorbed in
unchanged form after oral administration, the drug is widely believed to be
safe, with only flatulence as a commonly reported complaint. However, cases of
severe hepatotoxicity have been reported.10-12 Although
acarbose-induced hepatotoxicity appears to be uncommon, diabetic patients
receiving long-term acarbose therapy should be closely monitored for this
adverse effect.
Biguanides
Metformin
hydrochloride is widely used for the treatment of type 2 diabetes. A serious
but rare side effect, lactic acidosis, is caused because of its interference
with mitochondrial oxidative processes.13 Metformin (Fortamet,
Glucophage, Riomet) hepatotoxicity has rarely been reported, with two cases of
acute hepatitis and one of bland cholestasis.13-15 A
well-documented case of acute hepatitis caused by an idiosyncratic adverse
reaction to metformin or to one of its metabolites, has also been reported.
13
Thiazolidinediones
Thiazolidinediones
(TZDs, also known as glitazones) are insulin sensitizers now widely
used for the treatment of type 2 diabetes. Three TZDs have been used in
clinical practice: troglitazone, pioglitazone, and rosiglitazone.
Troglitazone:
Troglitazone (Rezulin), a peroxisome proliferators–activated receptor
gamma agonist that enhances insulin sensitivity, was approved
for the treatment of type 2 diabetes in 1997.16 It was
an effective antidiabetic drug with a fundamentally new mechanism
of action. However, within a year after its widespread use,
individual cases of liver injury and failure were reported.16-19
The mounting evidence for the idiosyncratic hepatotoxicity of
troglitazone in the following years led to its withdrawal from the
market in 2000.
Since then, a considerable
effort has been made to elucidate the mechanism of
troglitazone-induced hepatotoxicity. A number of hypotheses were
brought forward to explain troglitazone-induced cell injury,
including the formation and accumulation of toxic metabolites,
mitochondrial dysfunction and oxidant stress, inhibition of the
bile salt transporter and bile acid toxicity, and the induction of
apoptosis.17
Pioglitazone and Rosiglitazone:
After the withdrawal of troglitazone due to hepatotoxicity, only
pioglitazone (Actos) and rosiglitazone (Avandia) can be used for
the treatment of patients with type 2 diabetes. Fortunately, these two newer
drugs in the TZD class have a much larger margin of safety for liver toxicity.
Very rare reports of liver toxicity, usually milder and reversible, have been
seen with these drugs. Very few case reports have implicated it as a cause of
hepatocellular injury and granulomatous hepatitis.20-22 Severe
cholestatic hepatitis caused by rosiglitazone (8 mg/day) was reported in a
56-year-old female patient who had a history of receiving troglitazone
treatment; it is indicated that rosiglitazone is not always a safe alternative
in patients who had liver injury due to troglitazone.23
Pioglitazone-induced hepatocellular-cholestatic liver injury in a 49-year-old
patient with diabetes who was on this drug for six months. Liver enzyme values
returned to normal six weeks after the patient discontinued pioglitazone
therapy.24
In conclusion, while
pharmacovigilance for hepatotoxicity is probably still warranted, the
practitioner and patient can be fairly confident that these drugs are safe
from a liver standpoint. Finally, recent work would suggest that these agents
may prove useful to reduce hepatic fat in patients with nonalcoholic
steatohepatitis and may possibly protect against adverse metabolic
consequences and the ultimate development of cirrhosis in patients with fatty
livers.
Pioglitazone and rosiglitazone
are used either as monotherapy or in combination with metformin,
sulfonylureas, or glinides. The combination of TZDs with insulin is also
appealing, as it allows improvement of glycemic control while decreasing the
daily insulin requirement. Insulin dosage has to be adjusted regularly to
avoid hypoglycemic episodes. Recently, a prospective, open-labeled,
nonrandomized study was conducted to assess safety and efficacy of
rosiglitazone and insulin treatment in combination with poorly controlled
insulin-treated patients with type 2 diabetes.25 It was concluded
that the rosiglitazone plus insulin combination is safe and effective in this
population. However, further studies are warranted.
Sensitivity of
Hepatotoxicants in Diabetic Rodent Models
Hepatotoxicity of
several structurally and mechanistically diverse chemicals (i.e.,
acetaminophen; bromobenzene; carbon tetrachloride; chloroform; 1, 1,
2-trichloroethane; D-galactosamine; and thioacetamide) is significantly
altered in streptozotocin- or alloxan-induced type 1 diabetic rats.26-29
The majority of the studies have pointed out that hepatotoxicant-induced
liver injury is potentiated in type 1 diabetic rats. Similarly, hepatotoxicity
of allyl alcohol, bromobenzene, carbon tetrachloride, and thioacetamide have
been shown to potentiate in type 2 diabetic rats.30 Interestingly,
chemical-induced liver injury is differently modified by diabetes in murine
models. In contrast to the enhanced hepatotoxicity in diabetic rats, diabetic
mice (type 1 as well as type 2) are protected from normally lethal
hepatotoxicant challenge.31-34 Current understanding of altered
hepatotoxicity of xenobiotics in diabetes is summarized in Table 2.

Remarkable species differences
have been observed in rodent models. Diabetic rats exhibit marked sensitivity
versus diabetic mice exhibiting equally marked protection from drug-induced
hepatotoxicity. Availability of the rodent diabetic models offers a unique
opportunity to uncover mechanisms of clinical interest in averting human
diabetic sensitivity to drug-induced hepatotoxicities. While the rat diabetic
models appear to be suitable, the diabetic mouse models might not be suitable
in preclinical testing for potential hepatotoxic effects of drugs or
toxicants, because regardless of type 1 or type 2 diabetes, mice are resistant
to acute drug-or toxicant-induced toxicities.35
Conclusion
Recent
epidemiological studies suggested that patients with diabetes are twice as
likely to suffer hepatic failure compared to patients who do not have
diabetes. Increased incidences of hepatotoxicity have been observed in
patients with diabetes receiving drug therapies. Neither the mechanisms nor
the predisposing factors underlying hepatotoxicity in patients with diabetes
are clearly understood. While pharmacovigilance for hepatotoxicity is probably
still warranted, the practitioner and patient can be fairly confident that
these drugs are safe from a liver standpoint. However, it is recommended that
liver enzymes such as alanine amino transferase should be monitored in
patients with diabetes receiving antidiabetic drugs for which incidences of
hepatotoxicity are already reported.
REFERENCES
1. Centers for
Disease Control and Prevention. National Diabetes Fact Sheet. United States,
2005. www.cdc.gov/diabetes/pubs/pdf/ndfs_2005.pdf. Accessed February 12, 2008.
2. World Health Organization. Diabetes fact sheet, No. 312. September 2006. www.who.int/mediacentre/factsheets/fs312/en/index.html. Accessed February 12, 2008.
3. National Diabetes Information Clearinghouse. National diabetes statistics. http://diabetes.niddk.nih.gov/dm/pubs/statistics/index.htm#7. Accessed February 12, 2008.
4. Heron MP, Smith BL. Death: leading causes for 2003. Natl Vital Stat Rep. March 2007;55:1-92.
5. El-Serag HB, Everhart JE. Diabetes increases the risk of acute hepatic failure. Gastroenterology. 2002;122:1822-1828.
6. El-Serag HB, Tran T, Everhart J. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468.
7. Nakao NL, Gelb AM, Stenger RJ, Siegel JH. A case of chronic liver disease due to tolazamide. Gastroenterology. 1985;89:192-195.
8. Sitruk V, Mohib S, Grando-Lemaire V, et al. Acute cholestatic hepatitis induced by glimepiride. Gastroenterol Clin Biol.2000;24:1233-1234.
9. Heurgue A, Bernard-Chabert B, Higuero T, et al. Glimepiride-induced acute cholestatic hepatitis. Ann Endocrinol. 2004;65:174-175.
10. Madonia S, Pietrosi G, Pagliaro L. Acarbose-induced liver injury in an anti-hepatitis C virus positive patient. Dig Liver Dis. 2001;33:615-616.
11. Hsiao SH, Liao LH, Cheng PN, Wu TJ. Hepatotoxicity associated with acarbose therapy. Ann Pharmacother. 2006;40:151-154.
12. Mennecier D, Zafrani ES, Dhumeaux D, Mallat A. Acarbose-induced acute hepatitis. Gastroenterol Clin Biol. 1999;23:1398-1399.
13. Deutsch MM, Kountouras D, Dourakis SP. Metformin hepatotoxicity. Ann Intern Med. 2004;140:W25.
14. Babich MM, Pike I, Shiffman ML. Metformin-induced acute hepatitis. Am J Med. 1998;104:490-492.
15. Desilets DJ, Shorr AF, Moran KA, Holtzmuller KC. Cholestatic jaundice associated with the use of metformin. Am J Gastroenterol. 2001;96:2257-2258.
16. Watkins PB. Idiosyncratic liver injury: challenges and approaches. Toxicol Pathol. 2005;33:1-5.
17. Chojkier M. Troglitazone and liver injury: in search of answers. Hepatology. 2005;41:237–246.
18. Faich GA, Moseley RH. Troglitazone (Rezulin) and hepatic injury. Pharmacoepidemiol Drug Saf. 2001;10:537-547.
19. Chan KA, Truman A, Gurwitz JH, et al. A cohort study of the incidence of serious acute liver injury in diabetic patients treated with hypoglycemic agents. Arch Intern Med. 2003;163:728-734.
20. Al-Salman J, Arjomand H, Kemp DG, Mittal M. Hepatocellular injury in a patient receiving rosiglitazone. Ann Intern Med. 2000;132:121-124.
21. Forman LM, Simmons DA, Diamond RH. Hepatic failure in a patient taking rosiglitazone. Ann Intern Med. 2000;132:118-121.
22. Dhawan M, Agrawal R, Ravi J, et al. Rosiglitazone induced granulomatous hepatitis. J Clin Gastroenterol . 2002;34:582-584.
23. Bonkovsky HL, Azar R, Bird S, et al. Severe cholestatic hepatitis caused by thiazolidinediones: risks associated with substituting rosiglitazone for trolitazone. Dig Dis Sci. 2002;47:1632-1637.
24. May LD, Lefkowitch JH, Kram MT, Rubin DE. Mixed hepatocellular–cholestatic liver injury after pioglitazone therapy. Ann Intern Med. 2002;136:449-452.
25. Garg R, Gopal J, Jones GR. Rosiglitazone: safety and efficacy in combination with insulin in poorly controlled type 2 diabetes mellitus patients treated with insulin alone. J Diabetes Complications. 2007;21:1-6.
26. Aniya Y, Ojiri Y, Sunagawa R, et al. Glutathione S-transferases and chloroform toxicity in streptozotocin-induced diabetic rats. Jpn J Pharmacol. 1989;50:263-269.
27. Hanasono GK, Witschi H, Plaa GL. Potentiation of the hepatotoxic responses to chemicals in alloxan-diabetic rats. Proc Soc Exp Biol Med. 1975;149:903-907.
28. El-Hawari AM, Plaa GL. Potentiation of thioacetamide-induced hepatotoxicity in alloxan- and streptozotocin-diabetic rats. Toxicol Lett. 1983;17:293-300.
29. Wang T, Fontenot RD, Soni MG, et al. Enhanced hepatotoxicity and toxic outcome of thioacetamide in streptozotocin-induced diabetic rats. Toxicol Appl Pharmacol. 2000;166:92-100.
30. Sawant SP, Dnyanmote AV, Shankar K, et al. Potentiation of carbon tetrachloride hepatotoxicity and lethality in type 2 diabetic rats. J Pharmacol Exp Ther. 2004;308:694-704.
31. Gaynes BI, Watkins JB 3rd. Carbon tetrachloride and the sorbitol pathway in the diabetic mouse. Comp Biochem Physiol B. 1989;94:213-217.
32. Jeffery EH, Arndt K, Haschek WM. The role of cytochrome P450IIE1 in bioactivation of acetaminophen in diabetic and acetone-treated mice. Adv Exp Med Biol. 1991;283:249-251.
33. Shankar K, Vaidya VS, Wang T, et al. Streptozotocin-induced diabetic mice are resistant to lethal effects of thioacetamide hepatotoxicity. Toxicol Appl Pharmacol. 2003;188:122-134.
34. Sawant SP, Dnyanmote AV, Mitra MS, et al. Protective effect of type 2 diabetes on acetaminophen-induced hepatotoxicity in male Swiss-Webster mice. J Pharmacol Exp Ther. 2006;316:507-519.
35. Wang T, Shankar K, Ronis MJ, et
al. Mechanisms and outcomes of drug- and toxicant-induced liver toxicity in
diabetes. Crit Rev Toxicol. 2007;37:413-459.
To comment on this article,
contact rdavidson@jobson.com.





