ABSTRACT: Prostate cancer is the second leading cause of cancer death in men. Even with current therapies, many patients develop metastatic castration-resistant prostate cancer. The prognosis for these patients is poor, and there is a need for therapies to manage this population. Of the different approaches used to develop novel therapies, immunotherapies have shown the most promising results in trials and are currently being studied further for greater efficacy. Keeping abreast of these new developments will allow pharmacists to optimize drug use and improve patient regimens.
Prostate cancer is the second most common cancer in men worldwide and the most common cancer diagnosed in North American men, excluding skin cancers.1,2 At present, it accounts for 21% of all male cancers and 8% of male cancer-related deaths, making it the second leading cause of cancer death in men, exceeded only by lung cancer.3 The American Cancer Society predicts that in 2016, approximately 180,890 new cases and 26,120 prostate cancer–related deaths will occur in the United States.3
Since the introduction of the prostate-specific antigen (PSA) test in 1987, the incidence rates of prostate cancer have seen an increase, likely due to earlier detection.4 The rates in African American males are almost twice as high as those in whites, and more than a third of cases are diagnosed in men aged >75 years.3
Interestingly, the 5-year survival rates for patients diagnosed in the U.S. from 2001 to 2007 with local or regional disease have been as high as 100%, whereas for those with distant disease they are 28.7%.3 These figures stress the importance of early and accurate diagnosis and effective treatment.
Where Does the Pharmacist Fit in?
As the role of pharmacists evolves to a patient-centered, outcomes-oriented practice, there is a clear opportunity for pharmacists to make a significant impact in prostate cancer.4 In oncology, pharmacists are involved in medication reviews, therapeutic drug monitoring, supportive care counseling, elaboration of therapeutic guidelines, and the optimal use of resources. In order to optimize drug use and improve drug regimens, pharmacists need to keep up to date with the new drugs that are continually being developed.5 Complex regimens utilizing highly toxic agents are employed in the management of cancer patients. To ensure effectiveness of these agents while maintaining patient safety, pharmacists need to be familiar with the drugs employed.6
The introduction of oral chemotherapy as a noninvasive option for patients with metastatic castration-resistant prostate cancer (mCRPC) brings about further challenges for pharmacists. Oral chemotherapy agents have regimens that have novel toxicity profiles and require strict adherence for optimal therapeutic effects, frequent monitoring of laboratory parameters, monitoring for drug interactions, and proper handling and disposal methods.7,8
The most recent research has been in the field of immunotherapy and how agents that act on the immune system can be used to manage prostate cancer. The remainder of this article focuses largely on the few immunotherapy agents that have shown promising results in patients with mCRPC.
Treatment of Prostate Cancer
Although the precise etiology of prostate cancer has not been completely defined, hereditary and environmental factors have been shown to play a part in its process. Studies have shown that inflammatory processes and immune destruction are essential underlying components of its etiology.9,10
A range of agents and techniques are currently employed in the management of prostate cancer. The selection of the ideal agent is based upon the patient’s life expectancy, comorbidities, biopsy grade (Gleason score), clinical stage, and PSA level.10
The initial management of low-risk prostate cancer begins with watchful waiting and active surveillance. Surgical treatment or radiotherapy is used to manage early-stage prostate cancer, resulting in about 80% of men living free from metastatic disease for 15 years.11 As the disease progresses to late-stage prostate cancer, the aim of therapy is to reduce tumor burden and/or the level of testosterone through radiotherapy, surgery, and/or androgen deprivation using either surgical or chemical castration.12 Medical hormonal therapy traditionally involves the use of luteinizing hormone-releasing hormone (LH-RH) agonists or antiandrogens given alone or in combination. Eventually, the disease progresses further, resulting in castration-resistant prostate cancer, which typically requires the use of IV antineoplastic agents to extend overall survival, mitigate pain associated with the disease, and improve quality of life.8 The median survival in patients with mCRPC ranges between 12.2 and 21.7 months.13
The guidelines for patients with symptomatic mCRPC recommend the use of systemic chemotherapy, particularly docetaxel-based regimens, second-line hormonal therapy, bisphosphonates, or radioisotope therapy.14 Docetaxel has been shown to confer a median survival benefit of 2 to 3 months as compared to mitoxantrone and prednisone.13 Two new drugs, abiraterone and cabazitaxel, have been introduced for patients who are resistant to docetaxel.12 The prognosis for these patients is still poor, however, and a variety of other approaches are being tested with varying success.
Immunotherapy
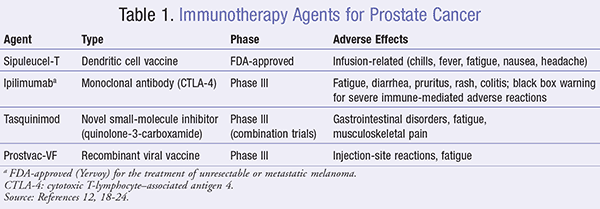
A number of studies support the use of agents that augment immune responses to prostate cancer, and research into many different immunotherapeutic strategies is under way (TABLE 1). This includes vaccines and agents that modulate T-cell activity, both as single agents and in combination with chemotherapy, androgen ablation, or radiotherapy.12,15
Dendritic Cell Vaccines
Therapeutic cancer vaccines are targeted nontoxic therapies that aim to induce durable tumor-specific immunity in patients with prostate cancer.16 They are used to present single or multiple tumor antigens to the immune system, via a variety of delivery systems, in order to prime or boost an immune response.17 They have emerged as a viable and promising treatment for mCRPC, and increasing evidence suggests that vaccine therapies are more effective when administered in the earlier stages of the disease.15
In 2010, sipuleucel-T (Provenge) was the first therapeutic cancer vaccine to receive FDA approval in the U.S.12 The approval was based upon a series of phase III trials including a pivotal 512-patient study, the IMPACT trial, which showed a statistically significant survival advantage with men in the active treatment group as compared to the placebo group.18 Sipuleucel-T is a personalized active cellular immunotherapy consisting of autologous peripheral-blood mononuclear cells (PBMCs), including antigen-presenting cells (APCs), that is manufactured using the patient’s own leukocytes.13 At a specialized facility, the process of leukapheresis is used to remove the leukocytes, which are then enriched for APCs, T cells, B cells, and natural killer (NK) cells. The APCs are activated with a recombinant fusion protein (PA2024).13 PA2024 fuses prostatic acid phosphatase (PAP) with granulocyte-macrophage colony-stimulating factor (GM-CSF), an immune cell activator. The resulting sipuleucel-T is returned to the clinic to be administered to the patient. The normal course of therapy is three doses given at 2-week intervals.19
Since the administration of sipuleucel-T varies from that of conventional drugs, pharmacists should explain the process to patients to enhance adherence. Furthermore, patients should be made aware that while sipuleucel-T improves the overall survival, it might not affect the PSA or radiographic response. For this reason, the progression of the disease needs to be monitored through serial imaging examinations. Studies have shown that the greatest survival benefit is seen in patients with the lower disease burden and, therefore, pharmacists should encourage patients to attend regular screenings for early detection and effective management.19
Most adverse events associated with sipuleucel-T are infusion-related and include chills, fever, fatigue, nausea, and headache, although these are typically low grade, transient, and can be managed in an outpatient setting.19 Treatment should be withdrawn in patients who experience an anaphylactic reaction.
Immune Checkpoint Inhibitors
Histologic data show a presence of CD4+ and CD8+ T cells, NK cells, dendritic cells, and macrophages within tumors. Furthermore, high-grade prostate cancer tumors have significantly lower infiltration of T cells, leading to the proposal that the progression of the tumor might be associated with defects in cell-mediated immune responses.9 The T-cell response against cancer can be inhibited by the upregulation of cell surface molecules on activated T cells.18 These molecules prohibit T cells from proliferating and exerting antitumor effector function. Based upon the fact that a high prevalence of regulatory T cells within tumors is associated with more lethal prostate cancer, studies have focused on the therapeutic blockade of the cells in an attempt to induce a beneficial clinical response.9
Ipilimumab is the first fully human monoclonal antibody that blocks cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) that potentiates an antitumor T-cell response. A pivotal phase III study demonstrated that the blockade of CTLA-4 with ipilimumab resulted in objective antitumor responses in approximately 11% of treated patients, with a disease control rate of about 29%.18 While clinical trials of ipilimumab have demonstrated some clinical activity in patients with mCRPC, the results have generally been disappointing.20
Tasquinimod is an oral quinolone-3-carboxamide that has shown antiangiogenic, antitumor, and immune-modulatory properties in preclinical models of prostate cancer and other solid tumors. In a large randomized phase II trial of tasquinimod in men with chemotherapy-naïve mCRPC, the median progression-free survival was doubled.9 The results of phase III trials were not as positive; while the drug significantly improved radio-graphic progression-free survival, this did not result in an overall survival benefit.21 Tasquinimod is currently under evaluation as a combination therapy with other systemic agents for the management of prostate cancer.22
Viral Vector Vaccines
Viral vectors mimic natural infection and can induce potent immune responses. Furthermore, a large number of tumor antigens and immunomodulatory genes can be inserted into them. This makes them useful vaccine vectors. Prostvac-VF is a recombinant viral vaccine engineered to contain PSA and three immune costimulatory molecules within a vaccinia or fowlpox virus vector.22 Vaccination is often enhanced by the subcu-taneous coadministration of GM-CSF, which acts to further boost immune function.
Prostvac-VF recently completed promising phase II trials, demonstrating a statistically significant improvement in overall survival of men with minimally symptomatic, chemotherapy-naïve mCRPC22; a large, global, phase III trial with overall survival as the primary endpoint is ongoing.23 The placebo-controlled phase II trial conducted on chemotherapy-naïve mCRPC patients resulted in a 44% reduction in death.22 Prostvac-VF is well tolerated and has been safely combined with other cancer therapies, including hormonal therapy, radiotherapy, another immunotherapy, and chemotherapy. In this regard, it holds great potential in the management of prostate cancer. As with sipuleucel-T, prostate cancer patients with earlier-stage disease and low-disease burden settings where immunotherapy can trigger a long-lasting immune response appear to achieve the optimal benefit from immunotherapy.24
Inhibitors of Prostate Cancer Cell Migration and Invasion
Another approach to the management of prostate cancer is to prevent the metastatic spread of tumor cells. Even though most of the prostate cancer–related deaths are due to the metastatic spread of tumor cells to distant organs, there is a lack of effective therapies to inhibit this process. One study group found that mitoxantrone hydrochloride, simvastatin, fluvastatin, and vandetanib inhibit both the migration and invasion of metastatic PC-3 prostate cancer cells without significantly affecting cell viability. The researchers propose further investigation of these drugs to reposition them for use in the management of prostate cancer.25
Conclusion
There is increasing interest in immunotherapies in cancer therapeutics following the recent approvals of drugs for the treatment of prostate cancer. These therapies can initiate an immune response that can kill tumor cells for an extended time after the conventional therapy has been administered. Even though the research in this field is very recent, promising trials have led to the development of molecules that may be used alone or in combination with other therapies (TABLE 1). In addition to improving the currently discovered molecules, further research needs to be conducted on the dosing and timing of administration of these agents as well as their use in combination with other therapies.
REFERENCES
1. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-1403.
2. National Cancer Institute. Prostate Cancer Screening (PDQ)—Health Professional Version. www.cancer.gov/types/prostate/hp/prostate-screening-pdq. Accessed July 1, 2016.
3. American Cancer Society. Cancer Facts & Figures 2016. Atlanta, GA: American Cancer Society; 2016. www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf. Accessed March 14, 2016.
4. Odedina FT, Warrick C, Vilme H, et al. Pharmacists as health educators and risk communicators in the early detection of prostate cancer. Res Social Adm Pharm. 2008;4(1):59-66.
5. Leveque D, Delpeuch A, Gourieux B. New anticancer agents: role of clinical pharmacy services. Anticancer Res. 2014;34(4):1573-1578.
6. Waddell JA, Solimando DA Jr. Cabazitaxel and prednisone regimen for prostate cancer. Hosp Pharm. 2015;50(6):460-463.
7. Holle LM, Puri S, Clement JM. Physician-pharmacist collaboration for oral chemotherapy monitoring: insights from an academic genitourinary oncology practice. J Oncol Pharm Pract. 2016;22(3):511-516.
8. Patel JM, Holle LM, Clement JM, et al. Impact of a pharmacist-led oral chemotherapy-monitoring program in patients with metastatic castrate-resistant prostate cancer. J Oncol Pharm Pract. 2015 Oct 22 [Epub ahead of print].
9. Silvestri I, Cattarino S, Aglianò AM, et al. Beyond the immune suppression: the immunotherapy in prostate cancer. Biomed Res Int. 2015;2015:794968.
10. Ghavamian R. Prostate cancer treatment protocols. Medscape. http://emedicine.medscape.com/article/2007095-overview. Accessed March 14, 2016.
11. Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580-593.
12. Gerritsen WR. The evolving role of immunotherapy in prostate cancer. Ann Oncol. 2012;23(suppl 8):viii22-viii27.
13. Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411-422.
14. Parker C, Gillessen S, Heidenreich A, Horwich A; ESMO Guidelines Committee. Cancer of the prostate: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(suppl 5):v69-v77.
15. Strauss J, Madan RA. Therapeutic vaccines for prostate cancer: recent advances and future directions. Expert Rev Vaccines. 2016;15(7):907-914.
16. Sonpavde G, Slawin KM, Spencer DM, et al. Emerging vaccine therapy approaches for prostate cancer. Rev Urol. 2010;12(1):25-34.
17. Palena C, Schlom J. Vaccines against human carcinomas: strategies to improve antitumor immune responses. J Biomed Biotechnol. 2010;2010:380697.
18. Antonarakis ES, Drake CG. Current status of immunological therapies for prostate cancer. Curr Opin Urol. 2010;20(3):241-246.
19. Pieczonka CM, Telonis D, Mouraviev V, et al. Sipuleucel-T for the treatment of patients with metastatic castrate-resistant prostate cancer: considerations for clinical practice. Rev Urol. 2015;17(4):203-210.
20. Wei XX, Fong L, Small EJ. Prospects for the use of ipilimumab in treating advanced prostate cancer. Expert Opin Biol Ther. 2016;16(3):421-432.
21. Mehta AR, Armstrong AJ. Tasquinimod in the treatment of castrate-resistant prostate cancer—current status and future prospects. Ther Adv Urol. 2016;8(1):9-18.
22. Singh P, Pal SK, Alex A, et al. Development of PROSTVAC immunotherapy in prostate cancer. Future Oncol. 2015;11(15):2137-2148.
23. U.S. National Institutes of Health. A randomized, double-blind, phase 3 efficacy trial of PROSTVAC-V/F +/- GM-CSF in men with asymptomatic or minimally symptomatic metastatic castrate-resistant prostate cancer (Prospect). NCT01322490. https://clinicaltrials.gov/ct2/show/NCT01322490. Accessed June 15, 2016.
24. Shore ND. PROSTVAC targeted immunotherapy candidate for prostate cancer. Immunotherapy. 2014;6(3):235-247.
25. Shah ET, Upadhyaya A, Philp LK, et al. Repositioning “old” drugs for new causes: identifying new inhibitors of prostate cancer cell migration and invasion. Clin Exp Metastasis. 2016;33(4):385-399.
To comment on this article, contact rdavidson@uspharmacist.com.






