US Pharm. 2024;49(11):31-35.
ABSTRACT: Breast cancer remains one of the most prevalent cancers in the United States. Outpatient oncology clinics and their growing role in chemotherapeutic care are a mainstay in improving treatment outcomes. A major role in these settings is played by oncology pharmacists, who manage and monitor chemotherapy regimens. Current management of breast cancer depends on identifying and treating each patient’s specific type of cancer. Newer oral agents expand the need for assistance with adherence to chemotherapy regimens, which broadens the scope of certified oncology pharmacists in the outpatient setting. Increasing adherence through managing adverse effects, preventing drug interactions, and providing patient education boosts favorable treatment outcomes.
In the United States, breast cancer is one of the top two cancers diagnosed in women, second only to nonmelanoma-type skin cancers.1 According to the American Cancer Society, it is predicted that one in eight women will be diagnosed with breast cancer and one in 39 women will die from breast cancer, or 13% and 3%, respectively.2 Factors that influence this likelihood include age, race, and ethnicity. Older age is associated with an increased incidence of breast cancer, and while white women have the highest incidence rate, increased mortality is seen in black women. This health disparity is attributed to later-stage diagnoses among black women.1,2 Treatment is often more effective and less extensive when an early diagnosis is made; in turn, outpatient oral chemotherapy is generally sufficient. When breast cancer is not detected until later stages, both a poor prognosis and decreased likelihood of survivability after diagnosis are more likely.2 Incorporating a variety of healthcare professionals is imperative to ensure collaboration in providing well-rounded patient care in outpatient oncology.
One way that treatment outcomes are enhanced is by including an oncology pharmacist on a multidisciplinary team. Oncology pharmacists, including board-certified oncology pharmacists (BCOPs), can manage aspects of therapy concerning chemotherapy for many types of cancer.3 Oncology pharmacists work alongside physicians and other healthcare providers to mitigate chemotherapy’s adverse effects, prevent potential drug-drug interactions, and alleviate patient medication concerns, among other responsibilities.4 Additionally, chemotherapy often has complex regimens, which makes it difficult for patients to maintain proper adherence. In the outpatient setting, oncology pharmacists can use pharmacotherapeutic knowledge to make interventions by providing patient education to promote medication adherence and satisfactory clinical outcomes.4,5 As the scope of oncology pharmacy increases with the expansion of treatment in the outpatient oncology setting, it can be expected that the role of pharmacists will become indispensable.
Clinical Management
Clinical management of breast cancer is determined by many factors, including genetics, cancer type, invasiveness, and quality of life. Generally, the majority of patients diagnosed with breast cancer will undergo some type of surgery to remove the affected tissue, and many of those patients will receive additional systemic therapies (e.g., radiation, chemotherapy, immunotherapy, as indicated) to best manage malignancies. The variety of breast cancer types and patient-specific factors make clinical management complex. As a result, a patient-centered, individualized approach is recommended for optimal outcomes.6
There are many types of breast cancer and patient profiles that must be considered when determining a treatment regimen; however, clinical practice guidelines can be generally applied to patients requiring treatment for specific breast cancers, such as malignant tumors that are classified as hormone receptor–positive (HR+), estrogen receptor–positive (ER+), and/or human epidermal growth factor type 2 receptor–positive (HER2+) or negative (HER2–). The 2024 Clinical Practice Guidelines by the National Comprehensive Cancer Network (NCCN) provide detailed treatment options for managing these types of breast cancer, including nonpharmacologic and pharmacologic management.6
Nonpharmacologic management of breast cancer is relatively limited in scope, as interventions are primarily based on lifestyle modifications that should be implemented during and after medical treatment. The NCCN guidelines highlight the association between healthy lifestyle habits and improved cancer outcomes.6 For example, patients with ER+ breast cancer who also developed a secondary tumor had an association with the following: obesity (BMI ≥30 kg/m2), smoking, and alcohol consumption. Additionally, patients who incorporated fruits and vegetables into their diet and exercised regularly were more likely to survive, even if they were considered obese. The NCCN recommends maintaining a healthy body weight (BMI 20-25 kg/m2) and implementing regular exercise to best improve outcomes during and after receiving therapy.6 Surgery is a common and often beneficial treatment for breast cancer, either as a primary intervention or in combination with another treatment, but its implementation is highly variable, as patients must qualify for and agree to surgery, and each patient’s cancer type and quality of life should be considered before proceeding. Therefore, surgery should be performed after the risks and benefits have been adequately assessed and discussed with the patient.6
Pharmacologic management of breast cancer is highly variable, so general principles guiding the clinical management of HR+, ER+, and HER2– tumor types will be described.6 The presence or absence of HR, ER, and HER2 in breast cancer tumors determines the optimal regimen for clinical management. Patients who are HR+ are often also ER+, so estrogen is commonly targeted in endocrine therapies.6,7 Although it is possible for some patients to be HR+, ER-negative (ER–), and progesterone receptor–positive (PR+) all at once (HR+/ER–/PR+), this is rare enough that the scope of the 2024 NCCN algorithm focused on patients who are HR+/ER+ and/or HR+/ER+/PR+.6 HER2+ patients should be treated to suppress HER2 to limit the growth and spread of cancerous cells. Patients who are ER–/PR– and HER2– have triple-negative breast cancer. Triple-negative cancer is harder to treat because it is not responsive to targeted therapies. As a result, patients who are HR+/ER+ and/or HER2+ tend to experience better clinical outcomes than patients who are triple negative.6,7
The NCCN guidelines recommend that patients who are HR+/ER+ receive endocrine therapy; however, the treatment algorithm for premenopausal and postmenopausal patients is different.6 Patients are generally classified as postmenopausal if they have not had a menstrual cycle for at least 12 consecutive months, except for patients who have undergone ovarian function–suppression therapy; in those patients, a postmenopausal classification is based on other criteria, such as hormone levels. Endocrine therapies for HR+/ER+ patients include tamoxifen and aromatase inhibitors (AIs; i.e., anastrozole, letrozole, or exemestane).6
It is recommended that premenopausal HR+/ER+ patients initiate either a 5-year course of tamoxifen or a 5-year regimen of AI therapy, with or without additional ovarian function–suppression therapy. Upon completing their initial regimens, premenopausal patients may continue another 5 years of tamoxifen or another 3 to 5 years of AI therapy, depending on the initial treatment; however, if they become postmenopausal after completing their initial therapy, they can continue another 5 years of tamoxifen or transition to 5 years of an AI.6
The primary recommended therapy for postmenopausal patients is an AI, although patients who have a contraindication to the medication class and/or are intolerant can use tamoxifen for 5 to 10 years, depending on clinical necessity. There are other factors that must be considered when postmenopausal patients initiate tamoxifen instead of an AI.6 For example, concomitant use of selective serotonin reuptake inhibitors can reduce the efficacy of tamoxifen, and anastrozole has been associated with better health outcomes in postmenopausal patients compared with tamoxifen.6,7 The recommended duration of therapy is contingent upon the initially selected regimen. Postmenopausal patients who start an AI for 5 years have the option to continue taking an AI for another 3 to 5 years, as clinically necessary. Alternatively, they may initiate an AI for 2 to 3 years and then transition to tamoxifen for 5 years. Patients who initiate tamoxifen for 2 to 3 years can switch to an AI for up to 5 years. Patients who complete a regimen of tamoxifen for 4.5 to 6 years can continue another 5 years of tamoxifen or transition to 5 years of an AI.6 TABLE 1 summarizes these recommendations.6
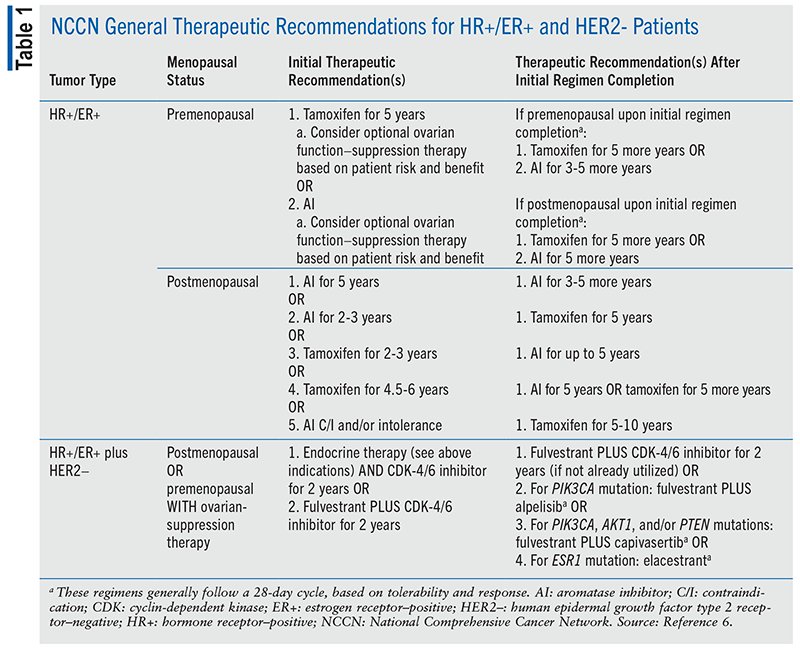
The NCCN guidelines recommend that patients who are HR+/ER+ and HER2– receive similar treatment, despite menopausal status, if premenopausal patients are receiving ovarian-suppression therapy.6 Recommended treatments for HR+/ER+/HER2– patients include endocrine therapy (i.e., an AI) plus a cyclin-dependent kinase (CDK)-4/6 inhibitor (i.e., abemaciclib, palbociclib, or ribociclib) for 2 years or fulvestrant plus a CDK-4/6 inhibitor for 2 years. Alternative therapies vary, as they are more individualized for each patient. Patients who complete initial endocrine therapy plus a CDK-4/6 inhibitor have the option to switch to fulvestrant plus a CDK-4/6 inhibitor for 2 years. Patients who are HR+/ER+ and HER2– may have additional tumor mutations that guide therapy. Patients who have a PIK3CA mutation should initiate fulvestrant plus alpelisib. Patients with a PIK3CA, AKT1, or PTEN mutation should initiate fulvestrant plus capivasertib. Patients with an ESR1 mutation should initiate elacestrant. The duration of therapy for each of the alternative regimens varies, as tumor response and tolerability influence clinical decision-making; however, the alternative regimens generally follow a 28-day cycle.6 TABLE 1 summarizes treatment recommendations for these patients.6
Newly Approved Agents for Outpatient Use
Oral chemotherapy is often used to treat breast cancer types, with medications such as tamoxifen and AIs being prescribed first-line.6 Since 2023, there have been five new oral chemotherapeutic agents approved by the FDA, resulting in oral formulations accounting for approximately 16% of the chemotherapeutic agents approved.8,9 Two of these five oral agents were approved for treatment of advanced or metastatic breast cancer. Truqap (capivasertib) was approved for treatment of HR+/HER2– advanced or metastatic breast cancer with the presence of PIK3CA, AKT1, and/or PTEN alterations following progression after at least one endocrine-based regimen or within 12 months of completing adjuvant therapy (in combination with fulvestrant). Capivasertib is an oral tablet taken twice daily. The most common side effects include decreased neutrophils, cutaneous adverse reactions, nausea, vomiting, diarrhea, fatigue, stomatitis, increased creatinine, increased triglycerides, and hyperglycemia associated with ketoacidosis. Patients with type 1 diabetes should use capivasertib with caution due to the risk of severe hyperglycemia and ketoacidosis. Capivasertib is also metabolized via the CYP3A4 pathway, so CYP3A4 inhibitors and inducers should be used with caution.10
Orserdu (elacestrant) was approved for men or postmenopausal women with ER+/HER2–, ESR1-mutated advanced or metastatic breast cancer with disease progression, following at least one endocrine-based regimen. Elacestrant is an oral tablet taken once daily. The most common side effects include musculoskeletal pain, complete blood count abnormalities, increased liver function enzymes, increased cholesterol and triglyceride levels, fatigue, hot flashes, dyspepsia, and gastrointestinal upset. Patients with a history of hyperlipidemia or liver dysfunction should use elacestrant with caution. Since elacestrant is also metabolized via the CYP3A4 pathway, CYP3A4 inhibitors and inducers should be used with caution.11
Oral chemotherapeutic agent regimens can be an appealing option to both patients and healthcare providers for many reasons. Compared with IV agents, oral agents can often be administered quickly and conveniently from the comfort of a patient’s home, without the need to travel to an infusion center or other healthcare facility. Additionally, patients with strictly oral chemotherapy regimens may be able to avoid getting a catheter port placed. Oral chemotherapy agents can also help decrease the transportation and time burden for both patients and healthcare providers by reducing the need for multiple hospital and/or clinic visits associated with IV chemotherapy. However, medication adherence may be a barrier for patients receiving oral chemotherapy at home without a healthcare provider’s supervision. Some of the most common reasons for oral chemotherapy nonadherence include adverse effects, lack of information about the treatment, and unintentional nonadherence (forgetting doses).12
Pharmacists can play an important role in ensuring that patients are adherent to their medications by educating patients about their medications and conditions, working with patients and healthcare teams to mitigate adverse effects, and counseling patients on strategies to minimize missed doses.
Board Certification Requirements for Oncology Specialization
Pharmacists can obtain board certification in their area of expertise through the Board of Pharmacy Specialties (BPS). A few examples of BPS specialties include oncology, pharmacotherapy, and infectious diseases. The standards and requirements for BPS certification are rigorous, and BPS-certified pharmacists can display an enhanced level of expertise in their practice, along with a more comprehensive approach to patient care. Additionally, they are often highly regarded in healthcare settings. BPS certification is often considered the gold standard for specializing in clinical pharmacy, and it offers a competitive edge for clinical pharmacists. Currently, there are approximately 4,310 BCOPs.13
To receive BCOP certification, pharmacists must be eligible to sit for and successfully pass the BCOP examination administered by BPS. BPS examinations are designed for highly qualified and specialized pharmacists; testing eligibility is selective, and BPS examinations do not assess minimum competency. Currently, the BCOP certification examination has a pass rate of approximately 58%.14 Specific criteria demonstrating expertise in a pharmacist’s respective field must be met to be eligible to sit for a BPS examination. The requirements that pharmacists must meet to sit for the BCOP examination include graduating from an Accreditation Council for Pharmacy Education program or other qualifying program outside of the U.S. and a current, active license to practice pharmacy in the U.S. or other jurisdiction. In addition, pharmacists must have demonstrated extensive oncology practice experience by either practicing in oncology for 4 years, within the past 7 years, completing a postgraduate year (PGY)-1 pharmacy residency within the past 7 years plus at least 2 years of practice experience in oncology, or completing a PGY-2 pharmacy residency in oncology within the past 7 years.
After passing the BCOP examination, pharmacists retain their certification for 7 years. Maintaining a BPS certification requires time and financial commitment; this helps ensure that pharmacy specialists are practicing at the top of their profession. Within the 7-year certification cycle, pharmacists must complete 80 to 100 units of BPS-approved continuing pharmacy education and/or additional continuous professional development, depending on the year BPS certification was obtained. An annual fee of $125 is required to maintain certification, and a $400 recertification fee is due in year 7.13
Role of the Pharmacist
Many patients receiving chemotherapy will experience at least one adverse effect, with the most common being nausea and vomiting, fatigue, dry mouth, constipation, diarrhea, hair loss, and changes in taste.15 The severity of adverse effects of chemotherapy can significantly impact the patient’s quality of life. Pharmacists can help manage the severity of adverse effects associated with chemotherapy. This can be done through pharmacologic interventions, such as initiating a serotonin receptor antagonist for a patient experiencing nausea and vomiting or decreasing the dose of a patient’s antineoplastic agent.16 Pharmacists are accessible and highly knowledgeable regarding medications, so including them on an outpatient oncology healthcare team can help improve patients’ quality of life.16 Moreover, decreasing the magnitude and frequency of adverse effects can positively influence medication-adherence patterns.
In the OPTIMAL Breast Cancer Care trial, incorporating a pharmacist into the healthcare team reduced the time between diagnosis and treatment initiation.17 Pharmacists were able to interview patients to identify adherence barriers, counsel patients on adverse effects, and review treatment schedules to make necessary adjustments.17 Improving adherence may ultimately provide better outcomes for patients taking oral chemotherapy. Additionally, antineoplastic medications are associated with many drug interactions. For example, proton-pump inhibitors may reduce the efficacy of several oral antineoplastic medications, most notably tyrosine kinase inhibitors and immune checkpoint inhibitors.18,19 By conducting a comprehensive medication review, pharmacists can identify major drug-drug interactions that have the potential to cause harm to patients.20,21 Oncology pharmacists also provide supportive-care management, monitor laboratory values, and coordinate the provision of investigational drugs.22 Therefore, as the rate of outpatient oral chemotherapy increases, the role of the pharmacist in this setting will also increase. By reviewing health records and treatment regimens, as well as providing effective counseling, pharmacists can improve patient outcomes in the outpatient setting.
Conclusion
With the advancement of chemotherapy agents to treat breast cancer, oral chemotherapy remains favorable for a number of reasons. Not only is it convenient and easy to administer, but it can also contribute to a reduction in IV infusion center visits and inpatient stays, medication burden, and financial costs related to transportation and healthcare visits. Pharmacists have the knowledge and skills to improve health outcomes by providing patient-centered care and education to reduce nonadherence and medication side effects. Additionally, pharmacists can ensure that patients receive guideline-based therapy, screen for drug interactions, and educate healthcare providers on appropriate laboratory monitoring and dosing schedules.
REFERENCES
1. CDC. Breast cancer statistics. September 16, 2024. www.cdc.gov/breast-cancer/statistics/index.html. Accessed August 14, 2024.
2. American Cancer Society. Key statistics for breast cancer. January 17, 2024. www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html. Accessed August 14, 2024.
3. National Cancer Institute. NCI dictionary of cancer terms. www.cancer.gov/publications/dictionaries/cancer-terms. Accessed August 21, 2024.
4. Gatwood J, Gatwood K, Gabre E, Alexander M. Impact of clinical pharmacists in outpatient oncology practices: a review. Am J Health Syst Pharm. 2017;74(19):1549-1557.
5. Oliveira CS, Silva MP, Miranda ÍKSPB, et al. Impact of clinical pharmacy in oncology and hematology centers: a systematic review. J Oncol Pharm Pract. 2021;27(3):679-692.
6. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines) breast cancer version 4.2024. www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed October 14, 2024.
7. National Cancer Institute. Breast cancer treatment (PDQ)–health professional version. January 19, 2024. www.cancer.gov/types/breast/hp/breast-treatment-pdq. Accessed August 19, 2024.
8. FDA. Novel drug approvals for 2024. www.fda.gov/drugs/novel-drug-approvals-fda/novel-drug-approvals-2024. Accessed August 20, 2024.
9. FDA. Novel drug approvals for 2023. www.fda.gov/drugs/novel-drug-approvals-fda/novel-drug-approvals-2023. Accessed August 20, 2024.
10. Truqap (capivasertib) product information. Wilmington, DE: AstraZeneca Pharmaceuticals LP; November 2023.
11. Orserdu (elacestrant) product information. New York, NY: Stemline Therapeutics, Inc; January 2023.
12. Talens A, Guilabert M, Lumbreras B, et al. Medication experience and adherence to oral chemotherapy: a qualitative study of patients’ and health professionals’ perspectives. Int J Environ Res Public Health. 2021;18(8):4266.
13. Board of Pharmacy Specialties. Oncology pharmacy. www.bpsweb.org/oncology-pharmacy/. Accessed July 30, 2024.
14. Board of Pharmacy Specialties. Continuous testing 2023 BPS certification and recertification examination results. www.bpsweb.org/2024/01/31/continuous-testing-2023-bps-certification-and-recertification-examination-results/. Accessed August 20, 2024.
15. Altun İ, Sonkaya A. The most common side effects experienced by patients were receiving first cycle of chemotherapy. Iran J Public Health. 2018;47(8):1218-1219.
16. Fujii H, Ueda Y, Hirose C, et al. Pharmaceutical intervention for adverse events improves quality of life in patients with cancer undergoing outpatient chemotherapy. J Pharm Health Care Sci. 2022;8(1):8.
17. Patel JV, Hughes DM, Ko NY. OPTIMAL Breast Cancer Care: effect of an outpatient pharmacy team to improve management and adherence to oral cancer treatment. JCO Oncol Pract. 2023;19(3):e306-e314.
18. Raoul JL, Moreau-Bachelard C, Gilabert M, et al. Drug-drug interactions with proton pump inhibitors in cancer patients: an underrecognized cause of treatment failure. ESMO Open. 2023;8(1):100880.
19. Uchiyama AAT, Silva PAIA, Lopes MSM, et al. Proton pump inhibitors and oncologic treatment efficacy: a practical review of the literature for oncologists. Curr Oncol. 2021;28(1):783-799.
20. Riu-Viladoms G, Carcelero San Martín E, Martín-Conde MT, Creus N. Drug interactions with oral antineoplastic drugs: the role of the pharmacist. Eur J Cancer Care (Engl). 2019;28(1):e12944.
21. Lopez-Martin C, Garrido Siles M, Alcaide-Garcia J, Faus Felipe V. Role of clinical pharmacists to prevent drug interactions in cancer outpatients: a single-centre experience. Int J Clin Pharm. 2014;36(6):1251-1259.
22. Holle LM, Segal EM, Jeffers KD. The expanding role of the oncology pharmacist. Pharmacy (Basel). 2020;8(3):130.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.






