US Pharm. 2009;34(11):HS-20-HS-26.
The American Cancer Society has estimated that 57,760 new cases of kidney cancer and 12,980 deaths due to kidney cancer will occur in the United States in 2009. Included in these statistics are renal cell carcinoma (RCC) and transitional cell carcinoma of the renal pelvis. Kidney cancer is one of the 10 most common types of cancer in men and women, with men at higher risk than women. The overall lifetime risk of developing kidney cancer is approximately 1 in 75 (1.34%).1
RCC constitutes 90% of kidney cancers, and the peak incidence is in the sixth decade of life. The histology is primarily clear cell carcinoma (85%); the remaining 15% comprises papillary, chromophobe, and collecting-duct carcinomas. The incidence of RCC is increasing, and about 30% of patients present with metastatic disease. Risk factors include smoking, hypertension, obesity, cystic kidney disease, and genetic abnormalities. One genetic abnormality is the mutation of the von Hippel-Lindau tumor-suppressor gene often seen in clear-cell carcinomas.2
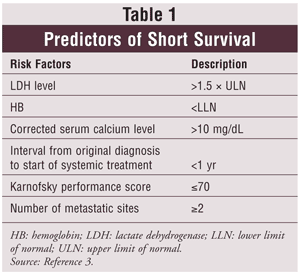
Patients with metastatic RCC have an estimated 10% survival rate at 5 years. A prognostic model to predict survival was developed to stratify patients into low-risk (no risk factors), intermediate-risk (1-2 risk factors), and poor-risk categories (>2 risk factors).2 See TABLE 1 for predictors of short survival.3
Current Treatment Options
Surgery is an important option for patients with RCC. Localized disease can be cured with surgery; unfortunately, about 30% of these patients will have disease recurrence. Surgery is a good choice for patients with advanced disease, benefiting quality of life by reducing pain or bleeding, and sometimes even improving survival.4
For many years, immunotherapy with the biologic response modifiers interleukin-2 (IL-2) and interferon-alpha (IFN-A) has been the mainstay of systemic treatment for metastatic disease. Treatment with these agents alone or in combination yields a median survival of 12 to 17.5 months.3 High-dose IL-2, which was FDA-approved in 1992 for advanced RCC, provides durable complete response in a small fraction of patients. Conventional chemotherapy yields response rates of less than 10% and often is used in a palliative context. High-dose nonmyeloablative chemotherapy followed by allogeneic stem-cell transplantation remains experimental.2,4
Targeted therapies have been developed to interfere with intracellular signaling, tumor-cell proliferation, differentiation, and angiogenesis. The small-molecule tyrosine kinase (TK) inhibitors bind to receptor TKs (RTKs) located on cell-surface growth factor receptors (GFRs), thereby inhibiting tumor growth.2 Sorafenib, which was approved in 2005 for advanced RCC, inhibits multiple intracellular and cell-surface kinases, including Raf; vascular endothelial GFR types 1 to 3 (VEGFR 1-3); platelet-derived GFR (PDGFR)-beta; the stem cell factor receptor (c-kit); Fms-like TK-3 (Flt3); and glial cell line-derived neurotrophic factor receptor (RET).4,5 Sunitinib, approved for advanced RCC in 2006, also inhibits multiple kinases, including VEGFR 1-3, PDGFR-alpha and -beta, c-kit, Flt3, colony-stimulating factor receptor type 1, and RET.4,6
Bevacizumab is a monoclonal recombinant humanized antibody that binds to and neutralizes circulating VEGF ligand, which prevents activation of the kinase VEGFR.2 It was FDA-approved in July 2009 in combination with IFN-A for patients with metastatic RCC. Clinical trials also demonstrate activity as single-agent therapy.4
Mammalian Target of Rapamycin (mTOR) Pathway
Three primary signaling pathways associated with RTKs are currently identified in cancer cells. One of them is the phosphoinositide 3-kinase (PI3K)/Akt/mTOR protein cascade (FIGURE 1); the other two are the protein kinase C family and the Ras/mitogen-activated protein kinase cascades. Activation of RTK leads to stepwise activation of PI3K, then Akt, and then mTOR, directly promoting tumor growth. The mutation and overexpression of RTK, as well as growth receptors, are common in RCC.7
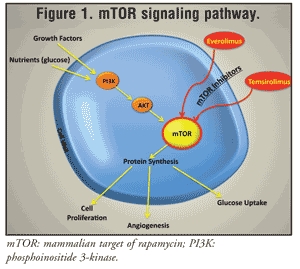
mTOR regulates essential signal-transduction pathways, tying growth stimuli to cell-cycle progression.7 It also integrates signals involving nutrient availability, energy status, and stress.8 There are two multiprotein complexes (mTORCs): mTORC-1 and mTORC-2. mTORC-1 is rapamycin-sensitive; mTORC-2 is rapamycin-insensitive.7
The mTOR pathway is important with regard to glucose and lipid metabolism. Interruption of the pathway by mTOR inhibitors increases the frequency of hyperglycemia and hyperlipidemia.7
mTOR Inhibitors
Rapamycin, also known as sirolimus, originally was developed as an antifungal drug, but it was initially used as an immunosuppressive agent in transplant patients because it inhibits T-cell proliferation after activation.9 In Europe, rapamycin also is used to prevent restenosis after percutaneous coronary interventions. Rapamycin was not studied for its anticancer activity until the late 1990s, when it was identified as the first mTOR inhibitor.7
To inhibit mTOR, rapamycin must bind to an abundant intracellular binding protein, FKBP-12; the drug:FKPB-12 complex binds at the rapamycin binding domain.8 The result of mTOR inhibition is cell-growth arrest, decreased protein synthesis, and reduced growth signaling. Rapamycin decreases the activity of mTORC-2 with sustained exposure.7
Rapalogues, or rapamycin derivatives, which were developed later, include temsirolimus and everolimus. The primary difference between the rapalogues is their pharmacokinetic and pharmacologic properties.7 Temsirolimus was created by adding an ester to a rapamycin carbon, and adding an ether created everolimus; the result was increased solubility and bioavailability.8 Notably, the rapalogues do not induce immunosuppression, as is seen with rapamycin.7,9
Temsirolimus
Temsirolimus, also known as CCI-779, is an IV-administered mTOR kinase inhibitor. The dosing is 25 mg once weekly as a 30- to 60-minute infusion. To prevent a hypersensitivity reaction, the patient should receive diphenhydramine 25 mg or 50 mg, or a similar antihistamine, about 30 minutes before the start of each dose. The dose should be held in the event of low neutrophil or platelet counts or severe adverse reactions.3,10
Temsirolimus binds to FKBP-12, forms a complex, and inhibits mTOR signaling. The agent inhibits cell division, halts growth signaling, and reduces hypoxia inducible factor (HIF-1 and HIF-2 alpha) and VEGF expression.3,10 It is hydrolyzed to the active metabolite sirolimus, which also likely inhibits mTOR. Temsirolimus has a terminal half-life of 12 to 15 hours; that of sirolimus is 40 to 50 hours.8 Temsirolimus is metabolized hepatically via CYP450 3A4. Drug interactions can occur with strong inducers and inhibitors, so these medications should be avoided. If coadministration is unavoidable, the dose of temsirolimus should be increased to 50 mg when given with a strong CYP450 3A4 inducer and reduced to 12.5 mg when given with a strong inhibitor.2 Grapefruit juice should be avoided because it may increase sirolimus levels. Use temsirolimus with caution in patients with hypersensitivity to polysorbate 80.10
Phase I and II clinical trials determined that temsirolimus 25 mg IV could be given safely on a weekly basis and had promising activity in RCC. Researchers also found that patients with treatment-refractory, poor-prognosis RCC who were receiving temsirolimus had a median overall survival of 8.2 months versus 4.9 months for IFN-A.7 Subsequently, Hudes and colleagues conducted a phase III trial comparing three treatment arms in 626 patients with metastatic RCC: temsirolimus 25 mg IV weekly; temsirolimus 15 mg IV weekly with IFN-A at 3 to 6 MU subcutaneously (SC) three times weekly; and IFN-A 3 to 18 MU SC three times weekly. Patients had to be treatment-naïve and have at least three predictors of short survival.3 See TABLE 2 for results.3
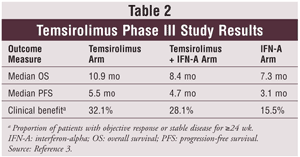
Dose reductions and delays were least common in the temsirolimus single-agent arm.3 The FDA approved temsirolimus for metastatic RCC in May 2007. As a result of the phase II and III trials, temsirolimus is now favored as first-line treatment for patients with poor-prognosis metastatic RCC.9
Everolimus
Everolimus, also known as RAD001, is an orally administered mTOR kinase inhibitor. It forms a complex with the intracellular protein FKBP-12 and inhibits the mTOR kinase. Everolimus reduces cell proliferation, cell growth, angiogenesis, and glucose uptake, and it inhibits HIF-1 expression and reduces VEGF expression.7,8,11
Everolimus is administered once daily as a 10-mg tablet, at the same time every day, with or without food. It is metabolized hepatically via CYP450 3A4. Drug interactions with CYP450 3A4 inducers and inhibitors should be avoided. If treatment with a strong CYP450 inducer cannot be avoided, the dose of everolimus may be increased from 10 mg to 20 mg in 5-mg increments. The dose of everolimus may also be reduced to 5 mg in the presence of moderate hepatic impairment or severe adverse events. The mean half-life is about 30 hours. There are six main metabolites of everolimus; they are 100 times less potent than the parent compound. Grapefruit juice should be avoided.11
Phase I and II studies established the safety and efficacy of everolimus administered on a daily oral dosing schedule. They also demonstrated disease stabilization or tumor reduction in metastatic RCC.4,7,9,12 Responding to the need for active cancer medications after traditional agents have failed, a phase III study investigated the role of everolimus in second- or third-line dosing.12
Motzer and colleagues conducted a phase III trial in 410 patients with metastatic clear cell RCC assigned in a 2-to-1 ratio to two treatment arms: oral everolimus 10 mg daily plus best supportive care or placebo plus best supportive care.12 Patients had to have progressed by or within 6 months of stopping sunitinib, sorafenib, or both drugs; previous therapy with bevacizumab, IL-2, or IFN-A was allowed. At progression, placebo patients could transition to active open-label everolimus treatment for ethical reasons. At the second interim safety analysis, the study was halted early because the prolongation in progression-free survival (PFS) was greater than expected. See TABLE 3 for results.12,13 The crossover from placebo to everolimus at disease progression would likely confound the median overall survival data for the everolimus group; therefore, the endpoint was not reached.12
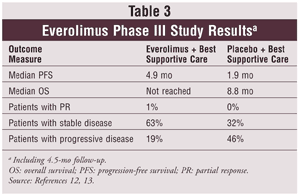
In a follow-up to the phase III everolimus study, researchers collected 4.5 months of additional blinded data for a total of 416 patients. The results of this study demonstrated persistence of PFS prolongation.13
In March 2009, the FDA approved everolimus for the treatment of advanced RCC after failure of treatment with sunitinib or sorafenib.11 The drug also is FDA-approved as a medical device as a component of the everolimus-eluting coronary stent system for coronary artery disease.14 Everolimus is approved in Europe as an immunosuppressant for the prevention of solid organ transplant rejection.9
Adverse Events
The primary adverse-events profile of the mTOR inhibitors includes hyperglycemia, hypercholesterolemia, and hyperlipidemia. This reflects the blockade of mTOR, the primary signaling conduit for insulin and insulin growth factor. Other common adverse events for temsirolimus and everolimus include fatigue, stomatitis, diarrhea, hypophosphatemia, low red blood cells and platelets, and peripheral edema. These adverse events are commonly reversible upon treatment discontinuation. Less common symptoms are renal insufficiency, interstitial pneumonitis, and low white blood cells.8
Fatigue is experienced by more than 50% of patients taking mTOR inhibitors. About 10% have dose-limiting fatigue to the degree of warranting dose reduction.9 Hyperglycemia is experienced by more than 50% of treated patients. Owing to the rising incidence of obesity and metabolic syndrome, it is important to identify these adverse events quickly. Up to 10% of patients develop severe symptoms and require medical intervention with an oral hypoglycemic agent or SC insulin. Regular monitoring of triglycerides is recommended, with early dietary interventions for minor elevations and initiation of a lipid-lowering agent for levels exceeding 500 mg/dL. Severe hypertriglyceridemia is associated with acute pancreatitis.9
Impairment of renal function and interstitial pulmonary fibrosis are rare but important events. Serum creatinine should be monitored regularly. The pulmonary fibrosis is often steroid-responsive and frequently fully reversible. If symptoms of progressive dyspnea or lung infiltrates are present, the dose may be reduced or the treatment discontinued.9
Both temsirolimus and everolimus are classified as pregnancy category D. Men and women receiving temsirolimus should use contraception while receiving active treatment and for 3 months after the final dose. Women taking everolimus should use contraception while receiving active treatment and for 8 weeks after the final dose.10,11
Patients being treated with mTOR inhibitors should not receive live vaccines. Patients should also avoid close contact with individuals who have received live vaccines.10,11
Investigational Combination Studies
The goal of combining different therapies is to achieve greater efficacy. Strategies often involve combining agents with different mechanisms of action and side-effect profiles. Investigational combinations in phase I and II studies include bevacizumab with either everolimus or temsirolimus; other studies are examining temsirolimus with sorafenib or IFN-A.4,8 Certain combinations require up-front dose reductions, such as temsirolimus with sorafenib, or are not safe, such as temsirolimus with sunitinib.15
Cost
The average wholesale price (AWP) of the temsirolimus injection kit 25 mg IV weekly is $1,499, or $5,996 per month. The respective monthly AWPs of everolimus 5 mg and 10 mg orally daily are $6,400 and $6,700. The manufacturers offer assistance programs for eligible patients. Prior authorization often is required for these high-cost medications.
Summary
There are two recently approved novel mTOR inhibitors with activity in metastatic RCC. Temsirolimus is FDA-approved for first-line treatment of metastatic RCC and is favored for poor-prognosis patients. Everolimus is FDA-approved for second-line treatment of metastatic RCC after sunitinib or sorafenib failure. These agents provide a valuable choice for providers and patients with limited treatment options.
REFERENCES
1. American Cancer Society. Detailed Guide: Kidney Cancer. What are the key statistics for kidney cancer? www.cancer.org/docroot/CRI/
2. Reeves DJ, Liu CY. Treatment of metastatic renal cell carcinoma. Cancer Chemother Pharmacol.
3. Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271-2281.
4. Basso M, Cassano A, Barone C. A survey of therapy for advanced renal cell carcinoma. Urol Oncol. Epub July 6, 2009. www.urologiconcology.org/ 2009;64:11-25.
5. Nexavar (sorafenib) package insert. Wayne, NJ: Bayer HealthCare Pharmaceuticals, Inc; February 2009.
6. Sutent (sunitinib) package insert. New York, NY: Pfizer Labs; July 2009.
7. Wysocki P. mTOR in renal cell cancer: modulator of tumor biology and therapeutic target. Expert Rev Mol Diagn. 2009;9:231-241.
8. Hudes GR. Targeting mTOR in renal cell carcinoma. Cancer. 2009;115(suppl 10):2313-2320.
9. Dasanu CA, Clark BA III, Alexandrescu DT. mTOR-blocking agents in advanced renal cancer: an emerging therapeutic option. Expert Opin Investig Drugs. 2009;18(2):175-187.
10. Torisel (temsirolimus) injection package insert. Philadelphia, PA: Wyeth Pharmaceuticals Inc; September 2008.
11. Afinitor (everolimus) package insert. East Hanover, NJ: Novartis Pharmaceuticals Corp; March 2009.
12. Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449-456.
13. Kay A, Motzer R, Figlin R, et al. Updated data from a phase III randomized trial of everolimus (RAD001) versus PBO in metastatic renal cell carcinoma (mRCC). Proc Am Soc Clin Oncol. 2009. Abstract 278 [presented at 2009 ASCO Genitourinary Cancers Symposium].
14. FDA. XIENCE™ V everolimus eluting coronary stent on the over-the-wire (OTW) or rapid exchange (RX) stent delivery systems-P070015. www.fda.gov/medicaldevices/
15. Sosman J, Puzanov I. Combination targeted therapy in advanced renal cell carcinoma. Cancer. 2009;115(suppl 10):2368-2375.
To comment on this article, contact rdavidson@jobson.com.





