US Pharm. 2021;46(1)22-26.
ABSTRACT: Neurocutaneous disorders are a diverse group of congenital disorders that encompass abnormalities of neuroectodermal and, sometimes, mesodermal development and often involve the skin, eye, and central nervous system. Tuberous sclerosis complex (TSC) and Sturge-Weber syndrome (SWS) are prototypical neurocutaneous disorders. Neurocutaneous disorders are often inherited conditions and typically present in early childhood or adolescence. There is an increased risk of neoplastic formation in many of the syndromes. Neurocutaneous syndromes are characterized by distinctive cutaneous stigmata and neurologic symptomology. Treatments are usually palliative rather than curative. Pharmacists can assist patients with medication therapy management for some symptoms of TSC and SWS.
Neurocutaneous disorders are a heterogeneous group of genetic disorders characterized by abnormalities of the cutaneous and nervous systems. Tuberous sclerosis complex (TSC) and Sturge-Weber syndrome (SWS) are prototypical neurocutaneous disorders in which genetic mutations in pathways regulating cell growth cause developmental dysfunction of the brain, skin, and other organs. Clinically, these neurocutaneous disorders differ significantly, but certain similarities exist. Namely, all neurocutaneous disorders are congenital, affect several organs, are associated with developmental problems, persist lifelong, and are currently uncorrectable. They are optimally managed with a multidisciplinary approach in which neurologists, oncologists, educational specialists, neuropsychologists, pharmacists, and other personnel work together to monitor for potential complications of the respective diseases and maximize the patient’s abilities. In addition, there is now emerging evidence of some overlap in the cellular signaling pathways in these disorders. There are around 30 to 40 types of neurocutaneous disorders.1,2
TUBEROUS SCLEROSIS COMPLEX
TSC is a multisystemic neurocutaneous genetic condition characterized by hamartomas that affect multiple organs, including the skin, central nervous system, heart, lungs, and kidney. Also known as epiloia or Pringle-Bourneville phacomatosis, TSC was initially described in the 19th century by Virchow and Von Recklinghausen, who identified hamartomas in the brain and heart during necropsy of patients with seizures and mental retardation. Campbell, in 1905, and Vogt, 3 years later, established the triad that characterizes TSC, which consists of mental retardation, epilepsy, and Pringle type of sebaceous adenoma (angiofibroma).3 The name tuberous sclerosis comes from the characteristic tuber or potato-like nodules in the brain, which calcify with age and become hard or sclerotic. The disorder affects as many as 25,000 to 40,000 in the United States and about 1 million to 2 million individuals worldwide, with an estimated prevalence of one in 6,000 newborns. It occurs in all races and ethnic groups and in both genders.4
Etiopathogenesis of TSC
TSC is characterized by autosomal-dominant mutations in the TSC1 or TSC2 genes (encoding for the protein Hamartin on chromosome 9q34 and Tuberin on chromosome 16q13 respectively) leading to overactivation of the mTOR (mechanistic target of rapamycin) pathway with increased cell proliferation and a range of other consequences.5 Individuals with TSC have a 50% chance of passing their condition on to each of their children. Familial cases of the condition are due to germline mutations, and despite the ability to be transmitted by heredity, 70% of TSC cases are the result of somatic mutations, configuring sporadic cases.6
Clinical Features of TSC
The clinical features of TSC are distinctive and can vary widely among individuals, even within one family. Major features of the disease include tumors of the skin, brain, kidneys, lungs, and heart, as well as seizures and TSC-associated neuropsychiatric disorders, which can include autism-spectrum disorder and cognitive disability.7,8
Skin lesions found in TSC include ash leaf spots, Shagreen patches, and adenoma sebaceum (multiple smooth papules that are benign angiofibromas). Hypomelanotic macules (ash leaf spots) are found in 90% of patients with TSC. Facial angiofibromas (benign tumors made up of blood vessels and fibrous tissues) are found in 75% of patients with TSC and are characterized by reddish spots or bumps on the face in a butterfly distribution. A Shagreen patch is an isolated raised plaque in the skin over the lower back or buttocks that is seen in 50% of affected children by adolescence. Ungual fibromas, small tumors under the toenails or fingernails, may also be present.9
Three types of brain lesions are seen in TSC: 1) cortical dysplasia, cortical tubers, and cerebral white matter radial migration lines (90%); 2) subependymal nodules (SENs), which are growths formed in the region surrounding the brain’s ventricles, called the subependymal zone (80%); 3) subependymal giant cell astrocytomas (SEGAs), slow-growing tumors of the brain (5% to 15%) that develop from SENs and grow such that they may block the flow of fluid within the brain, causing a buildup of fluid and pressure and leading to headaches and blurred vision.10-12
Renal disease is a leading cause of death in TSC patients. Angiomyolipomas (AMLs), benign tumors of fat and muscle tissues found in the kidneys, occur in 80% of TSC patients, and renal cystic disease occurs in 50% of patients. Both renal cystic disease and AMLs cause chronic renal disease, affecting approximately one million patients with TSC worldwide.10-12
Pulmonary involvement—specifically lymphangioleiomyomatosis (LAM), abnormal smooth muscle cells that grow out of control in lymphatic vessels and blood vessels in the lungs—is the third most common cause of TSC-associated morbidity, occurring in approximately 35% of female TSC patients. Dyspnea and recurrent spontaneous pneumothorax are the most common presentations, with slow and steady progression to respiratory failure.10-12
Cardiac rhabdomyomas, benign tumors of striated muscle, are found in 50% of people with TSC and are often detected on prenatal ultrasound. The majority of these lesions are asymptomatic and will spontaneously regress over time.10-12
Seizures and TSC-Associated Neuropsychiatric Disorders
Epileptic seizures are a hallmark of TSC and the most common clinical manifestation. The most prevalent seizure types in TSC are localization-related or focal seizures (67.5%), followed by epileptic (“infantile”) spasms in 38% to 49% of individuals with TSC. Epileptic spasms typically begin between the ages of 8 months and 4 years and later transform into other seizure types.13 Cognitive and behavioral impairments are also present. As many as 25% of TSC patients are autistic; more than half have learning difficulties. Aggressive and obsessive behaviors, as well as other psychological and psychiatric problems are also very common. Thirty percent to 40 % of people with TSC have profound mental retardation, with full-scale IQs of 40 or less. These persons are more likely to have a history of infantile spasms, intractable epilepsy, and seizure onset before age 1 year.14
Pharmacologic Management of TSC
The goals of treatment for individuals with TSC are to address the symptoms of the disorder, prevent loss of function of affected organ, improve quality of life, and reduce premature mortality and morbidity.
Tumor Reduction
Reduction in tumor volume is important in controlling symptoms of TSC. mTOR inhibitors, sirolimus (rapamycin), and everolimus (a rapamycin analogue) have successfully been used to treat renal angiomyolipomas and SEGAs in studies in TSC patients. Sirolimus also reduced lymphangioleiomyoma growth in some, but not all, patients.15
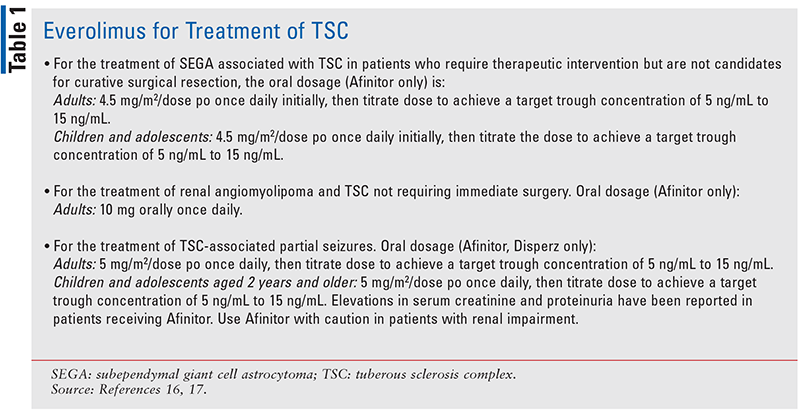
In one study, everolimus treatment resulted in a 30% or greater reduction in SEGA volume, a reduction in tuber (hamartomatous lesion) volume, improvement in facial angiofibromas, and an improvement in patient-reported seizure frequency over time (see TABLE 1 for dosing information).16,17
Sirolimus therapy in patients with TSC or sporadic LAM was associated with a reduction in angiomyolipoma volume of nearly 50% and, in the patients with LAM, improvements in airflow and gas trapping (measured as residual volume).
For the treatment of LAM, the recommended oral starting dose of sirolimus in adults is 2 mg po once daily; the maximum dosage for adults is 40 mg/day po. The renal and pulmonary benefits of treatment with sirolimus tended to reverse after the drug was withdrawn, though the improvements were persistent in some patients. Sirolimus monotherapy used in one trial resulted in a high rate of adverse events. Approximately 50% of patients had elevations in their serum lipid levels requiring dietary or pharmacologic intervention. Oral ulcers occurred to some degree in the majority of patients but typically resolved with topical therapy or a short-term reduction in sirolimus dose. Reported adverse effects of sirolimus include leukopenia, thrombocytopenia, hypertriglyceridemia, hypercholesterolemia, aphthous ulcers, edema, joint pain, interstitial pneumonia, delayed wound healing, and infection.18,19
Pharmacotherapy for Seizures and TSC-Associated Neuropsychiatric Disorders
Vigabatrin (oral) is the first-line therapy for treating infantile spasms, and adjunctive therapy for intractable partial seizures. Typical daily dosing starts at 25 mg/kg to 50 mg/kg with a typical maximum daily dose of 100 mg/kg to 150 mg/kg. Vigabatrin’s potential adverse effects include visual-field disturbances, insomnia, agitation, and constipation. Corticotropin (IM) 150 units/m2 and prednisone (oral) 2 mg/kg are second-line therapy for infantile spasms. Potential adverse effects of corticotropin and prednisone include hypertension, osteoporosis, gastric ulceration, and immunosuppression. Valproate or topiramate have efficacy in infantile spasms and may be added to vigabatrin on the uncommon occasion that infantile spasms prove refractory to vigabatrin monotherapy.20
Both oxcarbazepine and levetiracetam have been evaluated in the setting of TSC and partial seizures and have been well tolerated, even in some individuals responding to relatively high doses—greater than 60 mg/kg in the case of oxcarbazepine and greater than 100 mg/kg/day in the case of levetiracetam. Felbamate is a potent antiepileptic agent with primarily antiglutamatergic properties. It can be helpful for patients with TSC and partial seizures. The use of felbamate is hampered by risks of potentially irreversible hepatic failure and aplastic anemia.20
For aggressive behaviors not amenable to nonpharmacologic measures, atypical antipsychotic medications such as risperidone or quetiapine are useful. For obsessive and autistic behaviors, risperidone can be initiated at a low dosage (i.e., 0.125 mg/day to 0.25 mg/day) and titrated to optimal effect. Quetiapine (initial dosage of 12.5 mg/day to 25 mg/day) is more sedating and is preferred in patients in whom violent or aggressive behaviors predominate. Adverse effects with risperidone and quetiapine include weight gain (especially if combined with valproate), excessive sedation, constipation, and, rarely, ventricular arrhythmias.20
STURGE-WEBER SYNDROME
The Sturge-Weber syndrome (SWS), also known as encephalofacial angiomatosis, is a neurocutaneous disorder that occurs as a sporadic congenital condition; it is characterized by a port-wine stain (PWS) that affects the skin in the distribution of the ophthalmic branch of the trigeminal nerve and is associated with venous-capillary abnormalities of the leptomeninges and the eye. It occurs in both male and female newborns, in approximately one in 20,000 to 50,000 live births. A child born with a PWS on the face has approximately a 6% chance of having SWS, and this risk increases to 26% when the PWS is located in the distribution of the ophthalmic branch of the trigeminal nerve.21
Etiopathology of SWS
The pathology of the disease arises from the developmental disorder of all three germ layers (mesoderm, ectoderm, and neural crest). SWS and nonsyndromic PWS are caused by somatic activating mutations in the GNAQ gene located on chromosome 19q21, affecting early fetal vascular development. The GNAQ gene codes for the protein G alpha-q. When activated by the GPCR ligand, G alpha-q binds GTP and releases GDP, dissociates from the trimeric protein complex, and activates downstream pathways. The current understanding and data suggest that the mutation results in hyperactivation of downstream pathways, which include RAS-MEK-ERK, HIPPO-YAP, and, indirectly, mTOR.22
Clinical Features
SWS is characterized by the triad of facial capillary malformation (PWS), ocular choroidal hemangioma, and leptomeningeal (pial) angioma.23 In 1992, Roach categorized SWS variants into three types:
• Type I: individual has a facial PWS, leptomeningeal angioma, and may have glaucoma
• Type II: individual has a facial PWS, no leptomeningeal angioma, and may have glaucoma
• Type III: individual has leptomeningeal angiomatosis, no facial PWS, and, rarely, glaucoma.24
The most evident clinical manifestation is the presence of a cutaneous facial angioma, also known as the nevus flameus or PWS on the face, which normally follows the course of branches V1 and V2 of the trigeminal nerve. Hemiplegia and other neurologic disorders affect the opposite side of the nevus flameus.25,26
A leptomeningeal angioma, an abnormal leptomeningeal vascular malformation, typically occurs in the parietooccipital region and is ipsilateral to the PWS birthmark. However, intracranial involvement can be bilateral, and these individuals often manifest a more severe neurologic and developmental phenotype. The leptomeningeal vascular lesions disrupt normal cerebral blood flow and ultimately cause stasis as well as chronic insufficient tissue perfusion, resulting histologically in local brain atrophy due to gliosis, neuron loss, and calcification development.25
Ocular clinical manifestations of SWS include glaucoma. Ocular angioma, also present in the syndrome, appears in 30% of the cases, affects the choroids and the ocular sclera, and is ipsilateral to the cutaneous angioma. Vascular malformation problems in conjunctiva, episcleral vein, choroid, and retina, in addition to glaucoma, present in about 30% to 70% of the ophthalmic involvement cases.26
The neurologic complications of SWS include epilepsy; stroke-like episodes; migraine; and learning and behavioral difficulties affecting every aspect of the individual’s life. Epilepsy is very common in SWS, occurring in approximately 70% of those with unilateral leptomeningeal capillary malformation and 90% of those with bilateral disease, with most seizures presenting in the first 2 years of life.27
Nonpharmacologic Management of SWS
The PWS is treated with laser procedures beginning in infancy when the flat, pink birthmark responds best and is smaller. Early laser treatment may lessen later progression of the birthmark, which can consist of tissue hypertrophy, blebs, and complications affecting vision, airway, and swallowing.28
Pharmacologic Management
The main goal of glaucoma treatment is to control intraocular pressure (IOP) and to avoid progressive optic-nerve damage and visual-field loss. The increased IOP is treated with eye drops, such as timolol and latanoprost, which decrease fluid production in the eye. Beta-antagonist eye drops, adrenergic eye drops, and carbonic anhydrase inhibitors are the treatments of choice. The usual adult dose for latanoprost is 1 drop in the affected eye every day. Reduction of the IOP starts approximately 2 to 4 hours after the first administration, with the maximum effect reached after 12 hours. Individuals with asthma or severe COPD, or a serious heart condition (such as “sick sinus syndrome,” second or third degree atrioventricular block, severe heart failure, or very slow heartbeats) should not use timolol ophthalmic. Topical antiglaucoma drugs seem to be less efficacious in SWS patients with congenital glaucoma, although they represent first-line therapy for patients with late-onset glaucoma. One hypothesis for the low efficacy of antiglaucoma drugs in controlling SWS-related glaucoma is that most of these drugs do not affect episcleral venous pressure (EVP), highlighting the need for novel antiglaucoma medications specifically targeting EVP.28-30
Epilepsy in SWS can be difficult to control, occurring in clusters of seizures and episodes of status epilepticus. The most commonly used anticonvulsants in infants include oxcarbazepine, levetiracetam, and phenobarbital. A few patients develop infantile spasms, which may respond to steroids, topiramate, vigabatrin, or a ketogenic diet. Low-dose aspirin (3 mg/kg/day to 5 mg/kg/day) is also a therapeutic consideration, although not all groups use it. There is published evidence that it decreases the frequency and severity of stroke-like episodes and seizures. Side effects include increased bruising, nosebleeds, and gum bleeds; rarely, there are allergic reactions, and more seriously, bleeding can occur.28
CONCLUSION
Neurocutaneous syndromes are a diverse class of incurable congenital disorders. Routine monitoring of the skin, brain, organs, and eyes is necessary for long-term management of TSC and SWS. The pharmacist can play a role in helping to manage medications for seizures, tumor reduction, and associated neuropsychiatric disorders in TSC and glaucoma and epilepsy symptoms in SWS.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
REFERENCES
1. Klar N, Cohen B, Lin D, Neurocutaneous syndromes. Handb Clin Neurol. 2016;135:565-589.
2. Stafstrom CE, Staedtke V, Comi AM. Epilepsy mechanisms in neurocutaneous disorders: tuberous sclerosis complex, neurofibromatosis type 1, and Sturge-Weber syndrome. Front Neurol. 2017;8:87.
3. Portocarrero LKL, Quental KN, Samorano LP, et al. Tuberous sclerosis complex: review based on new diagnostic criteria. An Bras Dermatol. 2018;93(3):323-331.
4. National Institute of Neurological Disorders and Stroke. Tuberous sclerosis. Published 2007. https://catalog.ninds.nih.gov/pubstatic/07-1846/07-1846.pdf. Accessed October 18, 2020.
5. Ebrahimi-Fakhari D, Mann LL, Poryo M, et al. Incidence of tuberous sclerosis and age at first diagnosis: new data and emerging trends from a national, prospective surveillance study. Orphanet J Rare Dis. 2018;13(1):117.
6. Rodrigues DA, Gomes CM, Costa IM. Tuberous sclerosis complex. An Bras Dermatol. 2012;87(2):184-196.
7. Henske EP, Józwiak S, Kingswood JC, et al. Tuberous sclerosis complex. Nat Rev Dis Primers. 2016;2:16035.
8. Northrup H, Krueger DA. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49:243-254.
9. Little H, Kamat D, Sivaswamy L. Common neurocutaneous syndromes. Pediatr Ann. 2015; 44:496-504.
10. Radhakrishnan R, Verma S. Clinically relevant imaging in tuberous sclerosis. J Clin Imaging Sci. 2011;1:39.
11. Neumann HP, Schwarzkopf G, Henske EP. Renal angiomyolipomas, cysts, and cancer in tuberous sclerosis complex. Semin Pediatr Neurol. 1998;5:269-275.
12. Sasongko TH, Ismail NF, Zabidi-Hussin Z. Rapamycin and rapalogs for tuberous sclerosis complex. Cochrane Database Syst Rev. 2016;7(7):CD011272.
13. Zöllner JP, Franz DN, Hertzberg C, et al. A systematic review on the burden of illness in individuals with tuberous sclerosis complex (TSC). Orphanet J Rare Dis. 2020;15(1):23.
14. Franz DN, Bissler JJ, McCormack FX. Tuberous sclerosis complex: neurological, renal and pulmonary manifestations. Neuropediatrics. 2010;41(5):199-208.
15. Jülich K, Sahin M. Mechanism-based treatment in tuberous sclerosis complex. Pediatr Neurol. 2014;50(4):290-296.
16. Krueger DA, Care MM, Agricola K, et al. Everolimus long-term safety and efficacy in subependymal giant cell astrocytoma. Neurology. 2013;80(6):574-580.
17. Everolimus [2020]. Prescribers’ Digital Reference. PDR.net. https://www.pdr.net/drug-summary/Afinitor-everolimus-416.6101. Accessed December 22, 2020.
18. Bissler, J. McCormack F, Young L, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358:140-151.
19. Sirolimus [2020]. Retrieved from PDR.net. https://www.pdr.net/drug-summary/Rapamune-sirolimus-2097.4085. Accessed December 31, 2020.
20. Krueger D, Franz D. Current management of tuberous sclerosis complex. Paediatr Drugs. 2008;10:299-313.
21. Shirley MD, Tang H, Gallione CJ et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971-1979.
22. Comi AM, Sahin M, Hammill A, et al. Sturge-Weber syndrome research workshop. lLeveraging a Sturge-Weber gene discovery: an agenda for future research. Pediatr Neurol. 2016;58:12-24.
23. Warne RR, Carney OM, Wang G, et al. The bone does not predict the brain in Sturge-Weber syndrome. Am J Neuroradiol. 2018;39(8):1543-1549.
24. Shaikh SM, Goswami M, Singh S, Singh D. Sturge-Weber syndrome—a case report. J Oral Biol Craniofac Res. 2015;5(1):53-56.
25. Rosser T. Neurocutaneous disorders. Continuum (Minneap Minn). 2018;24(1, Child Neurology):96-129.
26. Palheta Neto FX, Vieira Jr MA, Ximenes LS, et al. Clinical features of Sturge-Weber syndrome, Int Arch Otolaryngology. 2008;12:4.
27. Luat AF, Juhász C, Loeb JA, et al. Neurological complications of Sturge-Weber syndrome: current status and unmet needs. Pediatr Neurol. 2019;98:31-38.
28. Comi A. Current therapeutic options in Sturge-Weber syndrome. Semin Pediatr Neurol. 2015;22(4):295-301.
29. Drugs.com. Timolol ophthalmic uses, side effects & warnings. Drugs.com. Accessed December 23, 2020.
30. Mantelli F, Bruscolini A, La Cava M, et al. Ocular manifestations of Sturge-Weber syndrome: pathogenesis, diagnosis, and management. Clin Ophthalmol. 2016;10:871-878.
To comment on this article, contact rdavidson@uspharmacist.com.