US Pharm. 2015;40(12):HS22-HS25.
ABSTRACT: Stress ulcer prophylaxis (SUP) has been a significant component in the management of critically ill patients. Antisecretory therapy, particularly proton pump inhibitors (PPIs), has been one of the most commonly utilized medications for SUP in hospital settings. However, several research studies have demonstrated that the overutilization of these agents has led to significant increases in hospital-related expenditures and adverse effects. Pharmacists play a unique role in improving the appropriate use and management of PPI therapy within the hospital setting.
Stress-related mucosal disease (SRMD), an acute erosive gastritis that may potentially lead to stress ulcers and/or stress-related injuries, remain a significant concern for patients within the critical care setting.1 The initiation of antisecretory therapy (AST) for prevention of stress ulcers within the critical care setting has gained widespread use and has been noted to protect against SRMD, which may lead to gastrointestinal (GI) bleeding.2 Although the use of these agents is well-established in the critical care setting, several research studies have confirmed the extensive and often inappropriate use of AST in both ICU and non-ICU patients, based on current recommendations.3
In the majority of hospital settings, proton pump inhibitors (PPIs) have been one of the most widely utilized drugs of choice for stress ulcer prophylaxis (SUP). The use of PPIs has risen dramatically since their introduction in the late 1980s, with sharp increases noted up to 456% in the 1990s.4 Among the world’s most frequently prescribed medications, annual expenditures for one branded PPI alone (esomeprazole [Nexium]) are estimated to be $6.1 billion, with 17.8 million prescriptions written.5 Although there is strong evidence demonstrating that SUP can be an effective preventive care measure, a perceived favorable safety profile and miseducation about appropriate indications of PPI therapy have contributed to the significant overuse of these agents.
Stress Ulcer–Related Risk Factors
Stress ulcers may present as a wide range of clinical manifestations that may be inclusive of single or multiple gastroduodenal mucosal defects and are a result of physiologic stress of serious illness.2 Clinical manifestations of stress ulcers may present as superficial mucosal erosions to more severe situations such as life-threatening bleeding.2,6,7 Although the risk of bleeding from stress ulcers appears to be on the decline, from 20% to 30% in the 1970s to 1.5% to 14% in the 1990s, mortality from stress-related bleeding in critically ill patients still approaches 50%.1,7 Multiple risk factors have been identified for the develop-ment of stress ulcers (TABLE 1).1,2,7,8

Patients within the critical care setting are at an increased risk of developing stress ulcers and subsequent GI bleeding as a consequence of both underlying illnesses and additional required therapeutic interventions, such as mechanical ventilation.2 A prospective multicenter cohort study found that respiratory failure (mechanical ventilation for at least 48 hours) and coagulopathy were strong independent risk factors for stress ulcer–related bleeding.8 The authors concluded that a limited number of critically ill patients present with clinically important GI bleeding, and SUP should be limited to those diagnosed with coagulopathy or whom require mechanical ventilation.8
Understanding Stress Ulcer Prophylaxis
The primary objective of SUP is to prevent stress ulceration by raising gastric pH >4.9 PPIs, potent gastric acid-suppressing agents, have increasingly replaced the use of histamine2-receptor antagonists (H2RAs) and sucralfate for SUP.9 The inhibition of gastric acid via the H+/K+-adenosine triphosphate pump (i.e., the proton pump) results in an increase of gastric pH above 6, which, in turn, is sufficient to prevent stress ulceration rebleeding.9 However, a limited number of studies have evaluated their effectiveness in comparison to other currently available antisecretory agents.
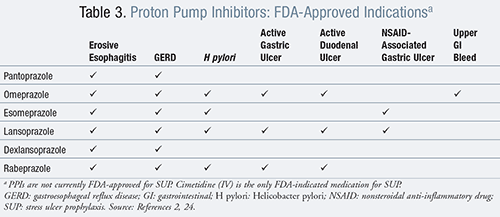
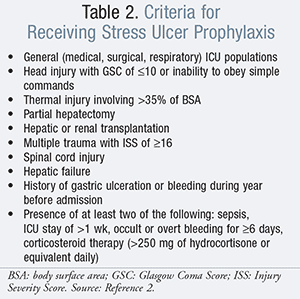
Currently, only one evidence-based guideline from the American Society of Health-System Pharmacists (ASHP) exists for SUP, which is aimed at discussing the use of SUP in critically ill patients exclusively.2 SUP is indicated in the following circumstances as listed in TABLE 2.2 However, it is important to note there is no inclusion for the use of PPIs as recommended therapy (TABLE 3). In addition, only IV cimetidine is FDA-approved for SUP in critically ill patients.2 However, a nationwide survey of critical care physicians revealed that 63.9% selected H2RAs as the first-line agent, followed by 23.1% selecting PPIs.10 Current guidelines indicate that SUP should not be recommended for medical or surgical patients who are not within a critical care setting.2,3
Clinical Research Supporting Overuse
Numerous research studies have indicated the excessive and often inappropriate use of PPI therapy within the hospitalized setting, leading to significant increases in hospital-related expenditures, patient-related adverse effects, and drug-drug interactions. Furthermore, in many instances, a significantly large percentage of patients who are prescribed a PPI either during their stay or upon discharge lacked appropriate indications.8,11,12
A university-affiliated community hospital sought to study the knowledge and attitudes of internal medicine residents toward SUP in noncritically ill patients.11 The authors concluded that SUP was prescribed to 74.1% of all patients during their hospital stay, with only 15% of patients meeting the appropriate indication for SUP.12
Pham et al conducted a retrospective chart review and reported that of the 213 patients examined, acid suppressant use increased following hospital admission to 71% (152 of 213), with 84% being prescribed PPIs, 11% H2RAs, and 5% combination therapy.11 Furthermore, only 10% (15 of 152) of those on acid suppressants were found to have an acceptable indication.11 For the 137 patients with nonaccepted indications, 29% had no evident indication and 38% were being prescribed corticosteroid-associated prophylaxis or SUP, although they were not needed.11
Leditschke et al evaluated the appropriateness of continued AST therapy. The authors noted that 81% of patients initiated on AST in a critical care setting were continued on the therapy after discharge to a general medicine ward.13 Only 29% of these patients had appropriate indications for acid suppression therapy.13
Nardino et al assessed patients admitted in a general medicine setting over three consecutive months.14 The authors determined that 55% of patients studied received AST for SUP, with 65% of listed indications being inappropriate for therapy.14 Inappropriate use included low-risk patients receiving prophylaxis and patients being discharged on AST who were originally started on AST during a previous hospital admission, and therapy was not needed.14
The inappropriate use of PPI therapy following hospital admission often leads to further issues upon discharge. A retrospective review of pharmacy claims data determined that out of 29,348 commercial and Medicare acute care study-eligible patients, 69% were inappropriately prescribed a PPI at discharge.15 Rates of inappropriate PPI utilization were statistically equivalent for ICU and non-ICU patients.15
Overall, there is considerable evidence demonstrating that PPIs are grossly overused despite no clear evidence that these agents are superior to H2RAs, sucralfate, or placebo. In several instances, PPIs were routinely utilized for SUP in non-ICU settings, although there was no justification for their use as determined by their admitting and/or other comorbidites.16
The Risks of Overuse
Adverse Effects: Inappropriate use of SUP is encumbered with major economic and patient safety implications, exposing patients to potential adverse effects such as increased risk of community-acquired pneumonia and Clostridium difficile–associated diarrhea (CDAD).17 Several studies have demonstrated evidence supporting the association between hospital-acquired CDAD infection and PPIs.18,19 Cunningham et al determined that PPI use within the preceding 8 weeks was associated with a 2.5-fold risk of CDAD.18 A case-control study of adult hospitalized patients showed that widespread use of PPI therapy for SUP appeared to contribute to an increased risk of C difficile infections (adjusted OR 2.75 [95% CI 1.68-4.52]; P = .0001).19
In recent years, the FDA has issued several warnings related to long-term use of PPIs. Postmarketing research prompted the agency to issue warnings of osteoporosis and fracture with the use of OTC PPI medications and decreased magnesium levels in patients prescribed PPIs for a year or longer.20
In addition, a recent study published in the Journal of General Internal Medicine sought to evaluate the net impact of PPIs on hospital mortality among medical inpatients.21 Through utilization of a computer-simulated model and literature-derived estimates of risk for upper GI bleeding, hospital-acquired pneumonia, and C difficile infections, the authors concluded that new initiation of PPI therapy led to an increase in hospital mortality in roughly 90% of simulated patients, secondary to the increased risk of infections in those utilizing acid suppression therapy.21 Although extrapolation of simulation study results to clinical practice may be limited, the research does provide reinforcing evidence that the risk versus benefit of PPI therapy should be evaluated prior to initiation.
Economic Impact: There are numerous economic and financial consequences associated with the inappropriate use of PPIs, with extensive costs shared between patients and insurance providers. Several studies have examined the economic impact of SUP overutilization in ICU patients.22,23 Erstad et al reviewed these costs and concluded that the per-patient drug cost approached $2,300 for inappropriate SUP.22 There was a 1.5-fold decrease in costs when providers received training in the appropriateness of SUP in ICU patients.22 Another clinical study determined that in non-ICU patients, an annual project cost savings between $26,366 and $35,456 may be achievable if efforts were made to limit inappropriate use and selection of appropriate route of administration of PPIs within their hospital.23
Role of the Pharmacist in Reducing Overuse
The use of PPIs in SUP is often routine practice within the critical care setting. However, its use poses various challenges that span not only to patients within the critical care setting but also following discharge. While the last ASHP guidelines for SUP were issued in 1999,2 clinical practitioners continue to anticipate the updated guidelines, which are slated for release in late 2015. Despite having an evidence-based guidance for the use of SUP, several studies have continued to demonstrate the abundant overuse of PPIs in the noncritical care setting, although research has revealed there is no benefit.9,11-14
Pharmacists may play a unique role in areas such as medication reconciliation upon transfer from critical care settings to general medicine units. Restricting the initiation of PPIs to the patients with the appropriate risk factors within both the critical care and general medicine setting is also an essential function in preventing overutilization, which pharmacists may perform. Pharmacists may also serve as key stakeholders in leading the coordination of care from the ICU or hospital to discharge, another important area in which removal of inappropriate PPIs should occur.3 The utilization of pharmacists in discharge planning is of optimal importance to ensure that all discharge medications are not only safe and efficacious, but also appropriate.
Education of medical staff is also a critically important aspect in reducing PPI overutilization. Needs-based prospective interventions, focused on involving faculty physicians and empowering senior medical residents, have proven efficacious in assisting prescribers to stop SUP if not indicated.12
Acknowledgments: The author would like to acknowledge the efforts and research contributions of Obi C. Okafor, Howard University College of Pharmacy Doctorate of Pharmacy candidate.
REFERENCES
1. Spirt MJ. Stress-related mucosal disease: risk factors and prophylactic therapy. Clin Ther. 2004;26(2):197-213.
2. ASHP therapeutic guidelines on stress ulcer prophylaxis. ASHP Commission on Therapeutics and approved by ASHP Board of Directors on November 14, 1998. Am J Health Syst Pharm. 1999;56(4):347-379.
3. Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5(4):219-232.
4. Guda NM, Noonan M, Kreiner MJ, et al. Use of intravenous proton pump inhibitors in community practice: an explanation for the shortage? Am J Gastroenterol. 2004;99(7):1233-1237.
5. Brown T. 100 most prescribed, best selling branded drugs through September. Medscape Medical News. November 3, 2014. www.medscape.com/viewarticle/834273. Accessed September 2, 2015.
6. Grube RR, May DB. Stress ulcer prophylaxis in hospitalized patients not in intensive care units. Am J Health Syst Pharm. 2007;64(13):1396-1400.
7. Quenot JP, Thiery N, Barbar S. When should stress ulcer prophylaxis be used in the ICU? Curr Opin Crit Care. 2009;15(2):139-143.
8. Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med. 1994;330(6):377-381.
9. Jung R, MacLaren R. Proton pump inhibitors for stress ulcer prophylaxis in critically ill patients. Ann Pharmacother. 2002;36(12):1929-1937.
10. Daley RJ, Rebuck JA, Welage LS, Rogers FB. Prevention of stress ulceration: current trends in critical care. Crit Care Med. 2004;32:2008-2013.
11. Pham CQ, Regal RE, Bostwick TR, et al. Acid suppressive therapy use on an inpatient internal medicine service. Ann Pharmacol. 2006;40(7-8):1261-1266.
12. Jain G, Jabeen SA, Vallurupalli S. Efforts to reduce stress ulcer prophylaxis use in non-critically ill hospitalized patients by internal medicine residents: a single-institution experience. J Clin Outcomes Manage. 2013;20(1):13-19.
13. Leditschke IA, Coombes JA. Concordance between use of proton pump inhibitors and prescribing guidelines. Med J Aust. 2000;172:564.
14. Nardino RJ, Vender RJ, Herbert PN. Overuse of acid-suppressive therapy in hospitalized patients. Am J Gastroenterol. 2000;95(11):3118-3122.
15. Thomas L, Culley EJ, Gladowski P, et al. Longitudinal analysis of the costs associated with inpatient initiation and subsequent outpatient continuation of proton pump inhibitor therapy for stress ulcer prophylaxis in a large managed care organization. J Manag Care Pharm. 2010;16(2):122-129.
16. Ramirez E, Lei SH, Borobia AM, et al. Overuse of PPIs in patients at admission, during treatment, and at discharge in a tertiary Spanish hospital. Curr Clin Pharmacol. 2010;5(4):288-297.
17. Heidelbaugh JJ, Inadomi JM, Goldberg KL. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk. Am J Gastroenterol. 2009;104(suppl 2):S27-S32.
18. Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhea. J Hosp Infect. 2003;54(3):243-245.
19. Jayatilaka S, Shakov R, Eddi R, et al. Clostridium difficile infection in an urban medical center: five-year analysis of infection rates among adult admissions and association with the use of proton pump inhibitors. Ann Clin Lab Sci. 2007;37(3):241-247.
20. FDA. Proton pump inhibitors information: current safety information. www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm213259.htm. Accessed August 15, 2015.
21. Pappas M, Jolly S, Vijan S. Defining appropriate use of proton-pump inhibitors among medical inpatients. J Gen Intern Med. 2015 Nov 9 [Epub ahead of print].
22. Erstad BL, Camamo JM, Miller MJ, et al. Impacting cost and appropriateness of stress ulcer prophylaxis at a university medical center. Crit Care Med. 1997;25:1678-1684.
23. Nasser S, Nassif J, Dimassi H. Clinical and cost impact of intravenous proton pump inhibitor use in non-ICU patients. World J Gastroenterol. 2010;16(8):982-986.
24. Aciphex (rabeprazole) package insert. Charlotte, NC: FSC Pediatrics, Inc; April 2013. http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020973s032lbl.pdf. Accessed November 11, 2015.
To comment on this article, contact rdavidson@uspharmacist.com.