US Pharm. 2012;37(12):HS-12-HS-15.
ABSTRACT: Estimates point to 43,920 new cases of, and 37,390 deaths from, pancreatic cancer (PC) in the United States in 2012. The absence of early symptoms and lack of diagnostic testing contribute to late-stage diagnosis, leading to poor survival rates and highlighting the need for early detection and improved treatment. Surgical resection remains the only potentially curative option. Chemotherapy (ChT) and radiotherapy are adjuvant treatments that may provide some benefit; however, poor outcomes persist. Investigational options should be considered in all phases of disease management. There is currently no standard ChT regimen for the adjuvant treatment of PC. The pharmacist plays a key role in educating patients about the importance of regular health care visits, healthy lifestyle, medication adherence, safe administration of oral ChT agents, and management of adverse effects associated with ChT.
Pancreatic cancer (PC) is a devastating and lethal disease affecting one in 68 Americans.1 The incidence rate of PC has increased by 1.5% per year since 2004, and an estimated 43,920 new cases and 37,390 deaths from PC in the United States were expected to occur in 2012.2 The median age at diagnosis from 2005 to 2009 was 71 years; the median age at death was 73 years. One-year and 5-year relative survival rates are 26% and 6%, respectively. These statistics highlight an urgent need for early detection and improved treatment of PC.
Along with the rise in incidence, the mortality rate has been increasing by 0.4% per year since 2004.2 The absence of early symptoms and lack of diagnostic testing contribute to late-stage diagnosis and the high mortality associated with PC. Symptoms, if present, include weight loss, upper abdominal pain that may radiate to the back, glucose intolerance, and jaundice (from common bile duct obstruction).2 Tobacco use (cigarettes, smokeless tobacco) is a known risk factor for PC, and cigarette smokers have twice the risk of developing PC compared with nonsmokers. Other risk factors include a family history of PC or a personal history of pancreatitis, diabetes, or obesity. Dietary consumption of red meat and alcohol has been proposed (but not proven) in epidemiologic studies as a possible cause of PC. Other potential factors, such as Lynch syndrome (in which the patient has a higher-than-normal risk of developing certain types of cancer, often before age 50 years) and other genetic disorders, may be associated with PC.2,3
TREATMENT OPTIONS
Nonpharmacologic
Surgery is the only potentially curative option for PC. Unfortunately, only 15% to 20% of patients are candidates for surgical resection at diagnosis.4 Age, comorbidities, performance status, frailty, and tumor location and size should be considered when deciding whether a patient is eligible for surgery. Tumors are classified as resectable, borderline resectable, or unresectable (locally advanced or metastatic).5 Patients are selected for surgery based on their likelihood of achieving an R0 resection (negative surgical margin), the key criterion for eligibility.6,7 Borderline-resectable patients may undergo neoadjuvant chemotherapy (ChT) or chemoradiotherapy (CRT) to improve the odds of an R0 resection, or they may have surgery immediately.8
Neoadjuvant therapy is given before the primary treatment in order to shrink the tumor or improve primary (surgical) treatment effects.3 Adjuvant therapy, administered after the primary treatment, is intended to reduce the risk of recurrence. The role of adjuvant radiotherapy (RT) is not yet defined, but RT is usually given before or after systemic ChT.9 RT should be administered at a dose of 45 to 46 Gy, with high-energy photons to the tumor bed, surgical anastomoses, and adjacent lymph node regions, followed by 5 to 15 Gy to the tumor bed (with careful attention to small-bowel exposure).8 No studies have demonstrated the superiority of CRT administered before versus after ChT. However, if a patient has a margin-positive resection, initial CRT followed by systemic ChT is appropriate.9-11
Pharmacologic
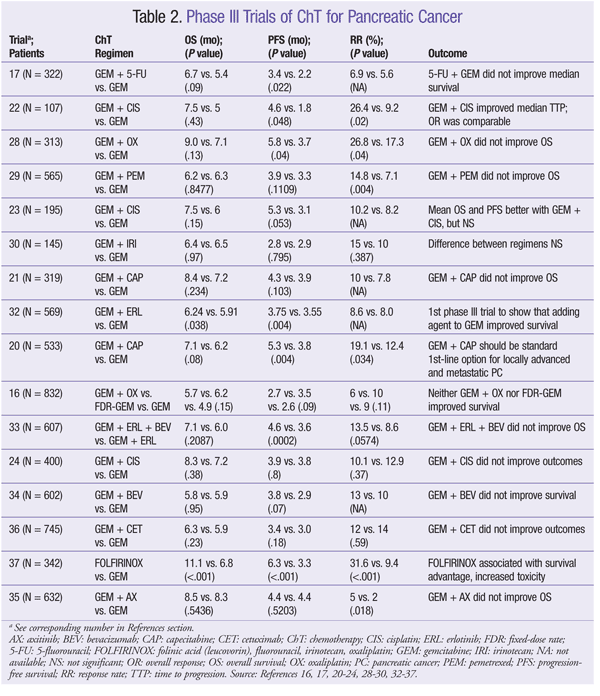
ChT has a modest effect against PC. However, there is an extremely high likelihood of a poor outcome, so investigational options should be considered in all phases of disease management.8 There is currently no standard combination ChT regimen for adjuvant treatment of PC, although several agents may be used. Pharmacology, dosing, and common adverse events (AEs) of ChT agents are given in TABLE 1. Outcomes of clinical trials involving ChT agents for PC appear in TABLE 2.
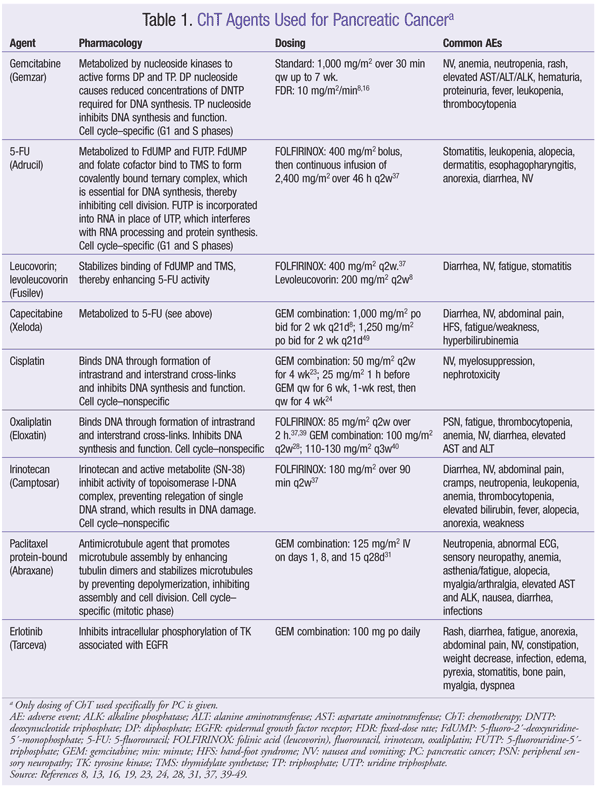
Gemcitabine (GEM): In 1997, GEM became the standard ChT regimen for advanced PC. In a randomized trial of GEM versus 5-fluorouracil (5-FU), GEM significantly improved median overall survival (OS) and clinical utility.12 GEM may be used as monotherapy in patients with metastatic disease and in patients with unresectable locoregional disease and good performance status, and for symptomatic relief in patients with metastatic or locally advanced unresectable disease and poor performance status.8 GEM should be given 1,000 mg/m2 over 30 minutes once weekly for up to 7 weeks (or until toxicity necessitates reducing/holding a dose), followed by a week of rest from treatment (TABLE 1).13
Fixed-Dose Rate GEM (FDR-GEM): Evidence supports the use of FDR-GEM for PC.14 One study found that patients given FDR-GEM had improved survival but more severe AEs, specifically hematologic toxicities.15 A subsequent trial demonstrated an increase in median OS with FDR-GEM versus standard infusion, although this did not satisfy protocol-specified criteria for superiority.16 FDR-GEM may be given at 10 mg/m2/min as an alternative to standard infusion over 30 minutes (TABLE 1).
GEM Combinations: Efforts to improve PC treatment have led to the investigation of GEM given together with various other ChT agents, but none of these combinations has demonstrated superiority over GEM monotherapy. To date, two phase III trials have compared GEM monotherapy with GEM in combination with 5-FU, but neither regimen increased OS.17,18 However, one of these trials showed improvement in progression-free survival (PFS) in patients with primarily metastatic disease.17 The combination of GEM and 5-FU may be used in patients with good performance status.
Capecitabine (CAP), an orally administered prodrug of 5-FU, mimics the continuous infusion of 5-FU.19 As with 5-FU, CAP given with GEM has not significantly improved OS compared with GEM alone.20,21 However, in one trial, the combination did improve PFS and response rate (RR) versus GEM alone.20 The combination of GEM and CAP is a viable option for patients with locally advanced or metastatic disease.8
GEM has also been investigated in combination with alkylating agents, with conflicting results. The combination of GEM and cisplatin (CIS) has not demonstrated an improvement in OS. Two phase III trials of GEM plus CIS showed a significant improvement in RR22,23; a different, larger study found no benefit in RR or PFS.24 GEM plus CIS may be a good choice for PC patients with certain family histories, such as BRCA mutation.25-27 Similarly, oxaliplatin (OX) in conjunction with GEM did not improve OS16,28; however, in one study, the combination improved PFS, RR, and clinical benefit.28 Improved OS has not been demonstrated for GEM combined with pemetrexed or irinotecan (IRI).29,30 Paclitaxel (protein-bound) plus GEM has shown activity against PC in a phase I-II trial and may be considered for patients with advanced disease and good performance status.31
GEM With Tyrosine Kinase Inhibitors (TKIs) and/or Monoclonal Antibodies (Mabs): GEM has been studied in combination with newer targeted ChT agents, such as TKIs and Mabs. GEM plus erlotinib (ERL) is the only combination that has shown an improvement in OS.32-36 In one study, GEM plus ERL significantly improved OS and PFS compared with GEM alone.32 Median survival favored the combination group (6.24 months in study group vs. 5.91 months in controls), and a higher percentage of patients survived at 1 year (23% in study group vs. 17% in control group).32 This combination is recommended for patients with locally advanced or metastatic disease and good performance status.8
Bevacizumab (BEV) combined with GEM plus ERL was studied, but this regimen did not improve OS.33 However, PFS was significantly improved compared with GEM plus ERL alone. The addition of BEV, axitinib, or cetuximab to GEM did not improve OS or PFS.34-36
Non-GEM Regimens: In the PRODIGE trial, FOLFIRINOX (folinic acid [LV], FU, IRI, OX) improved median PFS and median OS versus GEM.37 FOLFIRINOX has been made a category 1 recommendation for first-line treatment of good-performance-status patients with metastatic PC, and a category 2A recommendation for patients with locally advanced unresectable disease. AE incidence increased with the combination regimen, but these AEs did not significantly affect quality of life.37 CAP, 5-FU/LV/OX, and CAP plus OX are acceptable second-line options for metastatic PC.38-40
LV: There is currently an LV shortage in the U.S. Alternatives are to use levoleucovorin (TABLE 1); lower the LV dosage for all doses in all patients, which is likely to be just as efficacious; or increase the 5-FU dosage (10% range).8
TREATMENT GUIDELINES
Treatment of PC is determined by cancer staging of the cancer as resectable, borderline resectable, or unresectable or metastatic. TABLE 3 provides an overview of current treatment recommendations for pancreatic adenocarcinoma.

THE PHARMACIST’S ROLE
The pharmacist can play a vital role in educating patients about the necessity of routine visits to their health care provider, along with the importance of maintaining a healthy lifestyle. Smoking cessation is one of the most critical changes patients can make to improve their overall health and prevent the development of cancer and many other life-threatening diseases. Oral ChT agents such as CAP and ERL are becoming more prevalent in the management of cancer. Educating patients about medication adherence, safe administration of oral ChT agents, and AE management is fundamental to optimization of therapy. Finally, supportive care in patients receiving ChT is paramount. For more information regarding the National Comprehensive Cancer Network’s Guidelines for Supportive Care, visit www.nccn.org/professionals/physician_gls/f_guidelines.asp.
REFERENCES
1. National Cancer Institute. SEER stat fact sheets: pancreas.
http://seer.cancer.gov/statfacts/html/pancreas.html. Accessed October
26, 2012.
2. American Cancer Society. Cancer Facts & Figures 2012. Atlanta, GA: American Cancer Society; 2012:19.
3. NCI Dictionary of Cancer Terms. Neoadjuvant therapy. www.cancer.gov/dictionary?CdrID=45800. Accessed August 17, 2012.
4. Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049-1057.
5. Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment
of resectable and borderline resectable pancreatic cancer: expert
consensus statement. Ann Surg Oncol. 2009;16:1727-1733.
6. Talamonti M. Borderline resectable pancreatic cancer: a new classification for an old challenge. Ann Surg Oncol. 2006;13:1019-1020.
7. Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline
resectable pancreatic cancer: definitions, management, and role of
preoperative therapy. Ann Surg Oncol. 2006;13:1035-1046.
8. National Comprehensive Cancer Network. NCCN Clinical Practice
Guidelines in Oncology. Pancreatic adenocarcinoma. Version 2.2012.
www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed
October 25, 2012.
9. Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs
gemcitabine chemotherapy before and after fluorouracil-based
chemoradiation following resection of pancreatic adenocarcinoma: a
randomized controlled trial. JAMA. 2008;299:1019-1026.
10. Herman JM, Swartz MJ, Hsu CC, et al. Analysis of
fluorouracil-based adjuvant chemotherapy and radiation after
pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas:
results of a large, prospectively collected database at the Johns
Hopkins Hospital. J Clin Oncol. 2008;26:3503-3510.
11. Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy
and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience
(1975-2005). J Clin Oncol. 2008;26:3511-3516.
12. Burris HA III, Moore MJ, Andersen J, et al. Improvements in
survival and clinical benefit with gemcitabine as first-line therapy for
patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413.
13. Gemzar (gemcitabine) product information. Indianapolis, IN: Eli Lilly and Co; February 2011.
14. Grunewald R, Abbruzzese JL, Tarassoff P, Plunkett W. Saturation
of 2´,2´-difluorodeoxycytidine 5´-triphosphate accumulation by
mononuclear cells during a phase 1 trial of gemcitabine. Cancer Chemother Pharmacol. 1991;27:258-262.
15. Tempero M, Plunkett W, Ruiz Van Haperen V, et al. Randomized
phase II comparison of dose-intense gemcitabine: thirty-minute infusion
and fixed dose rate infusion in patients with pancreatic adenocarcinoma.
J Clin Oncol. 2003;21:3402-3408.
16. Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of
gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate
infusion) compared with gemcitabine (30-minute infusion) in patients
with pancreatic carcinoma E6201: a trial of the Eastern Cooperative
Oncology Group. J Clin Oncol. 2009;27:3778-3785.
17. Berlin JD, Catalano P, Thomas JP, et al. Phase III study of
gemcitabine in combination with fluorouracil versus gemcitabine alone in
patients with advanced pancreatic carcinoma: Eastern Cooperative
Oncology Group Trial E2297. J Clin Oncol. 2002;20:3270-3275.
18. Riess H, Helm A, Niedergethmann M, et al. A randomized,
prospective, multicenter, phase III trial of gemcitabine, 5-fluorouracil
(5-FU), folinic acid vs. gemcitabine alone in patients with advanced
pancreatic cancer. In: J Clin Oncol 2005 ASCO Annual Meeting Proceedings. 2005;23(suppl). Abstract LBA4009.
19. Xeloda (capecitabine) product information. South San Francisco, CA: Genentech USA, Inc; February 2011.
20. Cunningham D, Chau I, Stocken DD, et al. Phase III randomized
comparison of gemcitabine versus gemcitabine plus capecitabine in
patients with advanced pancreatic cancer. J Clin Oncol. 2009;27:5513-5518.
21. Herrmann R, Bodoky G, Ruhstaller T, et al. Gemcitabine plus
capecitabine compared with gemcitabine alone in advanced pancreatic
cancer: a randomized, multicenter, phase III trial of the Swiss Group
for Clinical Cancer Research and the Central European Cooperative
Oncology Group. J Clin Oncol. 2007;25:2212-2217.
22. Colucci G, Giuliani F, Gebbia V, et al. Gemcitabine alone or with
cisplatin for the treatment of patients with locally advanced and/or
metastatic pancreatic carcinoma: a prospective, randomized phase III
study of the Gruppo Oncologia dell’Italia Meridionale. Cancer. 2002;94:902-910.
23. Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III
trial of gemcitabine plus cisplatin compared with gemcitabine alone in
advanced pancreatic cancer. J Clin Oncol. 2006;24:3946-3952.
24. Colucci G, Labianca R, Di Costanzo F, et al. Randomized phase III
trial of gemcitabine plus cisplatin compared with single-agent
gemcitabine as first-line treatment of patients with advanced pancreatic
cancer: the GIP-1 study. J Clin Oncol. 2010;28:1645-1651.
25. Ferrone CR, Levine DA, Tang LH, et al. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol. 2009;27:433-438.
26. Oliver GR, Sugar E, Laheru D, Diaz LA. Family history of cancer
and sensitivity to platinum chemotherapy in pancreatic adenocarcinoma.
Paper presented at: 2010 ASCO Gastrointestinal Cancers Symposium;
January 22-24, 2010; Orlando, FL. Abstract 180.
27. Lowery MA, Kelsen DP, Stadler ZK, et al. An emerging entity:
pancreatic adenocarcinoma associated with a known BRCA mutation:
clinical descriptors, treatment implications, and future directions. Oncologist. 2011;16:1397-1402.
28. Louvet C, Labianca R, Hammel P, et al. Gemcitabine in combination
with oxaliplatin compared with gemcitabine alone in locally advanced or
metastatic pancreatic cancer: results of a GERCOR and GISCAD phase III
trial. J Clin Oncol. 2005;23:3509-3516.
29. Oettle H, Richards D, Ramanathan RK, et al. A phase III trial of
pemetrexed plus gemcitabine versus gemcitabine in patients with
unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639-1645.
30. Stathopoulos GP, Syrigos K, Aravantinos G, et al. A multicenter
phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine
(G) monotherapy as first-line treatment in patients with locally
advanced or metastatic pancreatic cancer. Br J Cancer. 2006;95:587-592.
31. Von Hoff DD, Ramanathan RK, Borad MJ, et al. Gemcitabine plus
nab-paclitaxel is an active regimen in patients with advanced pancreatic
cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548-4554.
32. Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine
compared with gemcitabine alone in patients with advanced pancreatic
cancer: a phase III trial of the National Cancer Institute of Canada
Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966.
33. Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trials of
bevacizumab in combination with gemcitabine and erlotinib in patients
with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231-2237.
34. Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus
bevacizumab compared with gemcitabine plus placebo in patients with
advanced pancreatic cancer: phase III trial of the Cancer and Leukemia
Group B (CALGB 80303). J Clin Oncol. 2010;28:3617-3622.
35. Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine
versus placebo plus gemcitabine in patients with advanced pancreatic
adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256-262.
36. Philip PA, Benedetti J, Corless CL, et al. Phase III study
comparing gemcitabine plus cetuximab versus gemcitabine in patients with
advanced adenocarcinoma: Southwest Oncology Group-directed intergroup
trial S0205. J Clin Oncol. 2010;28:3605-3610.
37. Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825.
38. Pelzer U, Kubica K, Stieler J, et al. A randomized trial in
patients with gemcitabine refractory pancreatic cancer. Final results of
the CONKO 003 study. In: J Clin Oncol 2008 ASCO Annual Meeting Proceedings. 2008;26(suppl). Abstract 4508.
39. Pelzer U, Schwaner I, Stieler J, et al. Best supportive care
(BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC
in patients for second-line advanced pancreatic cancer: a phase
III-study from the German CONKO-study group. Eur J Cancer. 2011;47:1676-1681.
40. Xiong HQ, Varadhachary GR, Blais JC, et al. Phase 2 trial of
oxaliplatin plus capecitabine (XELOX) as second-line therapy for
patients with advanced pancreatic cancer. Cancer. 2008;113:2046-2052.
41. Adrucil (fluorouracil) product information. Irvine, CA: Teva Parenteral Medicines, Inc; July 2007.
42. Leucovorin product information. Schaumburg, IL: APP Pharmaceuticals, LLC; December 2009.
43. Fusilev (levoleucovorin) product information. Irvine, CA: Spectrum Pharmaceuticals, Inc; April 2011.
44. Cartwright TH, Cohn A, Varkey JA, et al. Phase II study of oral
capecitabine in patients with advanced or metastatic pancreatic cancer. J Clin Oncol. 2002;20:160-164.
45. Cisplatin product information. Sellersville, PA: Teva Pharmaceuticals USA; February 2012.
46. Eloxatin (oxaliplatin) product information. Bridgewater, NJ: sanofi-aventis U.S. LLC; December 2011.
47. Camptosar (irinotecan) product information. New York, NY: Pharmacia & Upjohn Co; July 2012.
48. Abraxane (paclitaxel) product information. Summit, NJ: Celgene Corp; October 2012.
49. Tarceva (erlotinib) product information. Farmingdale, NY: OSI Pharmaceuticals, LLC; April 2012.
To comment on this article, contact rdavidson@uspharmacist.com.





