Osteoporosis, a term meaning “porous bones,” is a silent disease that occurs as a result of reduced bone formation and increased bone resorption.1 These factors compromise bone structure, leading to microfractures, which can later develop into clinical fractures.2 Complications of fractures include disability, body deformity, depression, pain, and death.2 Prevention of osteoporosis entails identifying secondary causes in order to modify risk factors, if applicable. Treating osteoporosis involves the use of prescription medications, calcium and vitamin D supplementation, and nonpharmacologic therapies, such as appropriate diet, exercise, and smoking cessation. The impact of a pharmacist’s knowledge of osteoporosis management can help ensure the proper use of medications, appropriate vitamin supplementation, and patient counseling on recommended lifestyle changes.
Prevalence
Osteoporosis is present in 44 million Americans, and the majority of this population is over 50 years of age.3 Women aged 50 years and older have a 50% chance of breaking a bone due to osteoporosis.4 To compare the incidence of other disease states with the prevalence of osteoporosis in women, the risk of hip fracture is more than the collective risk of uterine, ovarian, and breast cancer.4 Complications of hip fractures include an increased risk of future fractures and mortality. Within the first year of a hip fracture, the risk of mortality increases 10% to 20%, and future fracture risk multiplies by 2.5-fold.2 Therefore, the impact on the economy is evident, due to an increase in hospital admissions, physician appointments, and enrollment in nursing homes.2
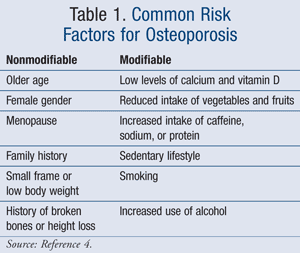
Risk Factors
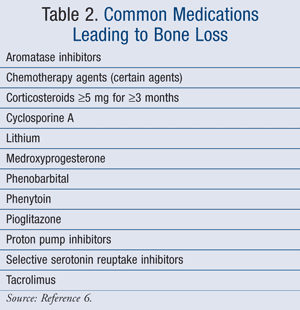
The leading causes of osteoporosis are separated into nonmodifiable and modifiable risk factors (TABLE 1).4 Women are more at risk for osteoporosis after menopause due to a reduction in estrogen, which plays an important role in bone protection.4 In addition, peak bone mass occurs between the ages of 18 and 25 years and slowly declines from this age onward.2 One study completed in 2007 specifically evaluated risk factors for osteoporosis in postmenopausal women.5 The results of this study demonstrated an increased risk of osteoporosis in women with a history of fracture, total body weight less than 127 lb, and the use of anticoagulants, such as warfarin or heparin. A reduction in the progression to osteoporosis was established in women with multiparity or a history of breastfeeding.5 In addition, certain medications can lead to bone loss (TABLE 2).6
Diagnosis
The diagnosis of osteoporosis is determined by measuring one’s bone mineral density (BMD), which serves as a predictor of future fracture risk. The National Osteoporosis Foundation (NOF) provides guidelines on the most up-to-date recommendations regarding diagnostic criteria (TABLE 3).2
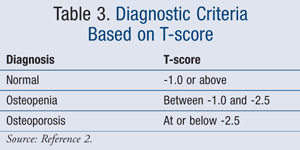
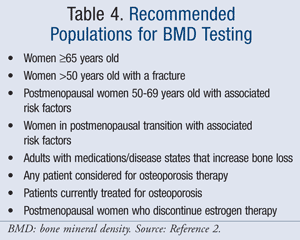
The World Health Organization (WHO) defines varying degrees of bone loss by T-scores.2 The significance of a T-score compares BMD to that of a “young normal” adult of the same gender. Additionally, the guidelines specify certain populations that are recommended to receive BMD testing (TABLE 4).2 A fracture risk calculator, known as FRAX, calculates the 10-year probability of a hip fracture and major osteoporotic fracture (available at www.shef.ac.uk/FRAX). This advanced tool provides the most accurate results in women with a low hip BMD.2
Pharmacologic Treatment
The NOF guidelines indicate which individuals should receive pharmacologic therapy for osteoporosis. Treatment for osteoporosis should be considered for postmenopausal women aged 50 years and older with any of the following: 1) fracture of the hip or vertebrae; 2) T-score less than or equal to -2.5; or 3) T-score between -1.0 and -2.5 and a 10-year risk of a hip fracture of at least 3% or a major fracture greater than or equal to 20% based on the WHO algorithm.2 In addition, therapy can be initiated based on patient preference, even if the patient does not meet the above criteria for treatment. The following medications are approved for treatment based on data from clinical trials demonstrating reductions in risk of fracture: bisphosphonates, calcitonin, selective estrogen receptor modulator (SERM), parathyroid hormone, and denosumab (TABLE 5).2 In addition, phase II and III trials of several new agents are underway to prove efficacy in fracture reduction.
Bisphosphonates are considered first-line therapy in the treatment of osteoporosis. The use of other FDA approved medications is considered if patients are intolerant to, have contraindications to therapy, or experience repeat fractures while using bisphosphonates. After treatment failure with bisphosphonates, the use of other pharmacologic therapies is based on the patient’s administration preference (i.e., oral vs. subcutaneous [SC]), adherence concerns, and safety of therapies.7
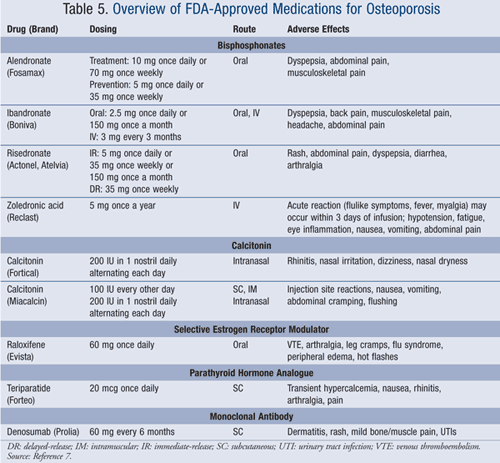
Bisphosphonates: The mainstay of osteoporosis therapy is bisphosphonates. Alendronate, ibandronate, risedronate, and zoledronic acid are FDA approved for the prevention and treatment of osteoporosis. Normal bone turnover involves a balance of bone formation (osteoblasts creating bone) and bone resorption (osteoclasts destroying bone); however, an imbalance (when bone resorption exceeds bone formation) can lead to bone loss and potential fractures. Bisphosphonates are antiresorptive agents; they inhibit bone resorption by inhibiting osteoclast activity.
Two landmark alendronate studies, the Fracture Intervention Trial (FIT) and the Fracture Intervention Trial Long-term Extension (FLEX), have been published.8,9 The FIT trial was a large, randomized study conducted in 6,549 women aged 55 to 80 years, with and without a history of vertebral fracture. Twelve to 18 months into the study, alendronate proved efficacious with overall reductions in hip fracture of 53% and clinical vertebral fracture of 45%. This trial provided evidence for the use of bisphosphonate therapy in reducing the risk of fracture for up to 5 years.8
The FLEX trial consisted of the same population from the FIT trial and studied the efficacy of continuing alendronate for another 5 years or discontinuing therapy after 5 years.9 Discontinuing alendronate therapy after 5 years did not significantly increase the risk of fracture, whereas subjects continuing alendronate therapy maintained mean serum bone turnover markers and BMD similar to baseline levels of the FIT trial. The use of bisphosphonates beyond 5 years is patient specific and appears to be most beneficial in patients with a high fracture risk.9
Proper administration of bisphosphonates is essential to improve absorption and reduce the risk of adverse effects. Alendronate and risedronate are taken first thing in the morning, with 6 to 8 ounces of water on an empty stomach. It is important for patients to remain sitting or standing and to avoid medications, eating, and drinking liquids, except water, after administration for the next 30 minutes. Ibandronate follows the same administration instructions, but requires a 60-minute time period before food intake and lying down.7 However, delayed-release risedronate (Atelvia), a new formulation, may provide patients a convenient alternative; it should be taken right after breakfast with 4 ounces of water once weekly. It should not be chewed, cut, or crushed. Patients need to wait 30 minutes before lying down and avoid taking concurrent medications and supplements.10
Zoledronic acid given as a yearly IV infusion can be used in patients who experience intolerable adverse effects or are unable to properly administer oral formulations.7 This medication was studied in the Health Outcomes and Reduced Incidence with Zoledronic Acid Once Yearly (HORIZON) Pivotal Fracture Trial, which compared zoledronic acid 5 mg IV infusion every 12 months with placebo for a total of 36 months.11 Zoledronic acid showed significant reductions in morphometric vertebral fractures by 70% and hip fractures by 41% compared with placebo.11
Bisphosphonate therapy should be avoided in patients with a creatinine clearance of less than 30 or 35 mL/min, depending on which agent.7
In October 2010, the FDA added the risk of atypical femur fractures to bisphosphonate labels.12 The two types of fractures observed under study were below the hip joint (subtrochanteric femur fracture) and at the long part of the thigh bone (diaphyseal femur fracture). In combination, these fractures are very uncommon and account for less than 1% of all hip and leg fractures.12 Patients should be educated to report any thigh or groin pain to their health care provider.
In July 2011, the FDA announced reported incidences of esophageal events and esophagitis with oral bisphosphonates.13 These events have been noted to occur in patients who do not use proper administration technique of the oral dosage form. To date, the FDA has not determined whether esophageal cancer risk is increased by oral bisphosphonate use.13 Patients are reminded to discuss the risks versus benefits of bisphosphonates and to contact their health care provider if they experience pain while swallowing, difficulty swallowing, or worsening heartburn.13
Calcitonin: There are two calcitonin agents (Fortical, Miacalcin) currently FDA approved for the treatment of osteoporosis in women who are postmenopausal for at least 5 years. Calcitonin is involved with calcium regulation and directly inhibits osteoclastic bone resorption.7 Both medications are generally safe and offer different routes of administration—intranasal and SC. A study published in 2000 compared the efficacy of alendronate versus calcitonin for osteoporosis in postmenopausal women. Increases in BMD and reductions in bone turnover were significantly greater at 12 months with alendronate compared to intranasal calcitonin. The results of this study further verify the use of bisphosphonates as first-line agents, but offer calcitonin as a therapeutic alternative for osteoporosis.14
Selective Estrogen Receptor Modulator (SERM): Raloxifene is the only SERM approved by the FDA for the prevention and treatment of osteoporosis in postmenopausal women. It is also indicated for breast cancer prevention. Raloxifene has similar effects as estrogen in preventing bone loss, but potentially antagonizes estrogen in uterine and breast tissue. In addition, raloxifene increases BMD and reduces bone resorption. Over 3 years, raloxifene has shown vertebral fracture reductions of 30% in patients with a prior fracture and 55% in patients without a previous fracture.2 Raloxifene has shown an increased risk of venous thromboembolism (VTE); therefore, its use is contraindicated in patients with a history of VTE.7
Parathyroid Hormone Analogue: Teriparatide is FDA approved for use in patients at high risk of fracture in postmenopausal women, men, and patients on prolonged glucocorticoid therapy.2 The mechanism of action is similar to that of the endogenous form of parathyroid hormone, which functions to promote osteoblast activity. In addition, the drug increases the absorption of calcium in the gastrointestinal tract and reabsorption through the renal tubules. Efficacy data at 18 months demonstrated a 65% reduction in vertebral fractures and a 53% reduction in nonvertebral fractures.2 Generally, this medication is well tolerated, but should be avoided in patients who are at an increased risk of osteosarcoma.7 Teriparatide is not recommended for use longer than 2 years, due to limited safety data. Limitations of therapy include daily SC injections into the abdomen or thigh and higher cost compared to other osteoporosis medications.7
Monoclonal Antibody: Denosumab is a new, fully human monoclonal antibody. This agent acts by binding to receptor activator of nuclear factor kappa-B ligand (RANKL), which is an important cytokine essential for the formation, activation, and survival of osteoclasts.15 Denosumab prevents the binding of RANKL to its receptor, RANK, on osteoclasts, which results in reversible inhibition of bone resorption by osteoclasts and increases BMD.15 In 2009, the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) trial was published.16 The objective of the study was to assess denosumab versus placebo over 3 years in the primary end point of reducing new vertebral fractures. Results demonstrated a 69% reduction in vertebral fractures and a 61% reduction in multiple new vertebral fractures with denosumab, which was statistically significant compared to placebo.16,17
The efficacy of this medication compared to existing therapies is limited, due to the lack of head-to-head studies. Denosumab is expensive compared to other therapeutic options, but an advantage is biannual SC injections. Overall, denosumab serves as a therapeutic option for patients who are unable to tolerate bisphosphonates due to esophageal irritation or for patients with low levels of adherence.16,17
Estrogen/Hormone Therapy (ET/HT): Based on the NOF guidelines, ET/HT is not approved for the treatment of osteoporosis. This statement is based on the associated increased risk of stroke, pulmonary emboli, myocardial infarction, breast cancer, or deep vein phlebitis with these agents. Although FDA approved indications include prevention of osteoporosis and alleviation of symptoms associated with menopause (i.e., vasomotor symptoms, vulvovaginal atrophy), ET/HT is not recommended as a first-line agent for osteoporosis prevention.2
Future Therapies: There are several agents for the management of osteoporosis completing phase II and phase III trials that employ different mechanisms of action compared with current therapies. Odanacatib, a phase III agent, inhibits cathepsin K, which is produced by osteoclasts and functions to degrade bone.17 Two anabolic agents studied for postmenopausal women with osteoporosis, MK-5442 and AMG 785, are undergoing phase II trials that are expected to be completed in 2012. MK-5442 targets the calcium-sensing receptor (CaSR) on the parathyroid gland.17 During hypocalcemia, the CaSR is activated, causing the release of parathyroid hormone, which increases calcium levels in the blood.17 MK-5442 is known as a calcilytic drug, due to its hypocalcemic mimicking properties.17 In addition, AMG 785 is a sclerostin monoclonal antibody that functions to improve bone formation.17
Nonpharmacologic Therapy and Calcium/Vitamin D Supplements
The use of nonpharmacologic therapy, such as diet, exercise, and vitamin supplements, is essential in preventing and treating osteoporosis. Women should consume a high intake of foods rich in calcium (e.g., low-fat dairy products) and vitamin D. Fortified milk, orange juice, cereals, and fish are examples of foods high in vitamin D. Exposure to sunlight, approximately 15 minutes daily, increases vitamin D synthesis in the body, leading to increased concentrations.7 Women with osteoporosis need to include at least 30 minutes of either aerobic or weight-bearing and muscle-strengthening exercise three times per week in their routine. Additionally, other modifiable risk factors need to be evaluated, such as smoking cessation, reducing alcohol intake, and incorporating vegetables and fruits into a daily diet.2 Fall prevention is equally important. The risk of falls can be minimized by regularly monitoring vision and hearing, clearing indoor and outdoor walkways, and limiting the use of medications that can affect balance, such as sedatives, anticholinergics, benzodiazepines, tricyclic antidepressants, and antihypertensives that cause hypotension.7
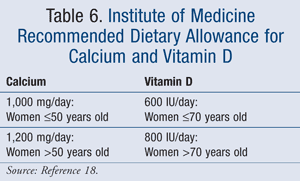
The NOF recommends at least 1,200 mg elemental calcium/day for postmenopausal women over 50 years of age and 800 to 1,000 IU/day for vitamin D in women 50 years of age and older.2 In addition, on November 30, 2010, the Institute of Medicine (IOM) released recommendations for the appropriate amount of calcium and vitamin D intake.18 The recommendations, which are based on data from more than 1,000 studies, concluded that appropriate levels of vitamin D and calcium are essential for bone growth and development. However, these supplements have no impact on other disease states, such as cancer, cardiovascular disease, or diabetes. The recommendations are based on Dietary Reference Intakes (DRIs), which are the total requirement from diet and dietary supplements (TABLE 6).18
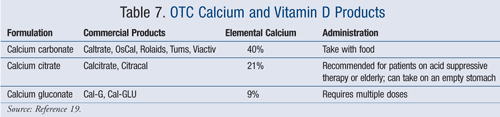
The amount of daily calcium required is based on the quantity of elemental calcium in each product. Maximal absorption of calcium occurs at 500 to 600 mg per dose.1 Calcium supplementation should be divided throughout the day, usually twice daily, to achieve maximal absorption. Each OTC product has varying amounts of calcium and vitamin D. In general, calcium carbonate products have a higher percentage of elemental calcium (TABLE 7).19 Common side effects are constipation, bloating, and flatulence. These products should be separated from certain medications such as iron, tetracyclines, and fluoroquinolones, because they can interfere with absorption.7
Monitoring
The gold standard of monitoring osteoporosis therapy is to evaluate BMD of the hip and spine by a central dual-energy x-ray absorptiometry (DXA) scan every 2 years and at the occurrence of clinical fractures.2 Frequent monitoring of medication safety is also important, and adverse effects are listed for each drug class in TABLE 5.
Conclusion and the Pharmacist’s Role
The best way to treat osteoporosis is to prevent its occurrence. Modification of risk factors is imperative, and pharmacists can play a large role in this area. Pharmacists can counsel patients—especially women, who are at an increased risk for osteoporosis—to increase their intake of foods and drinks rich in calcium and vitamin D; offer strategies to quit smoking; encourage 30 minutes of weight-bearing and muscle-strengthening exercise at least three times per week; and, most importantly, recommend appropriate calcium and vitamin D supplementation and any required prescription medications for osteoporosis. Pharmacists can determine the best therapeutic option for patients based on efficacy, tolerable adverse effects, contraindications, and medication adherence. Patients require counseling on proper dosing, administration, and adverse effects to promote the safe use and monitoring of medications for osteoporosis. Medication adherence and monitoring of BMD by central DXA is critical to reduce fracture risk and progression of osteoporosis. Refer to TABLE 8 for useful online resources for patients and pharmacists regarding osteoporosis management.

REFERENCES
1. Osteoporosis. Mayo Clinic.
www.mayoclinic.com/health/
2. National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2010.
www.nof.org/sites/default/
3. Qaseem A, Snow V, Shekelle P, et al. Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:404-415.
4. Factors that put you at risk. National Osteoporosis Foundation.
www.nof.org/node/51. Accessed March 22, 2011.
5. Schnatz PF, Marakovits KA, O’Sullivan DM. Assessment of postmenopausal women and significant risk factors for osteoporosis. Obstet Gynecol Surv. 2010;65:591-596.
6. Medicines that may cause bone loss. National Osteoporosis Foundation.
www.nof.org/node/232. Accessed March 22, 2011.
7. Phillips BB. Osteoporosis. In: Chisholm-Burns MA, Schwinghammer TL, Wells BG, et al, eds. Pharmacotherapy Principles & Practice. 2nd ed. New York, NY: McGraw Hill Medical; 2010:965-977.
8. Black DM, Thompson DE, Bauer DC, et al. Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. J Clin Endocrinol Metab. 2000;85:4118-4124.
9. Black DM, Schwartz AV, Ensrud KE, et al. Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927-2938.
10. Atelvia (risedronate sodium) package insert. North Norwich, NJ: Norwich Pharmaceuticals, Inc.; January 2011.
11. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809-1822.
12. FDA: possible increased risk of thigh bone fracture with bisphosphonates. FDA news release. October 13, 2010.
www.fda.gov/NewsEvents/
13. Oral osteoporosis drugs (bisphosphonates): drug safety communication—potential increased risk of esophageal cancer. FDA. July 21, 2011.
www.fda.gov/Safety/MedWatch/
14. Downs RW Jr, Bell NH, Ettinger MP, et al. Comparison of alendronate and intranasal calcitonin for treatment of osteoporosis in postmenopausal women. J Clin Endocrinol Metab. 2000;85:1783-1788.
15. Prolia (denosumab) package insert. Thousand Oaks, CA: Amgen Manufacturing Ltd; June 2010.
16. Cummings SR, San Martin J, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765.
17. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377:1276-1287.
18. Dietary reference intakes for calcium and vitamin D. Institute of Medicine of the National Academies. November 30, 2010.
www.iom.edu/Reports/2010/
19. Comparison of oral calcium salts. Pharm/Prescriber Letter. 2008;24:241008.
To comment on this article, contact rdavidson@uspharmacist.com.





