US Pharm. 2021;(46):18-22.
Infant mortality in the United States compares unfavorably with that of other countries. In 2018, the infant mortality rate in the U.S. was 5.7 deaths per 1,000 live births. The leading causes of infant deaths include birth defects, preterm birth, and maternal pregnancy complications. Many factors that contribute to poor pregnancy outcomes are already present at the time when prenatal care is initiated, because almost 45% of all pregnancies are unintended. Factors and exposures that can potentially affect fetal development include exposure to various medications, deficiency in folic acid, and uncontrolled chronic disease states. When treating women of childbearing age, it is important to manage them with the goal of optimizing control of chronic conditions and using pregnancy-friendly medications even if no pregnancy is planned for the immediate future.1-3
Preconception care has become a very important avenue to help reduce poor pregnancy outcomes. This care is defined as personalized care for men and women that is focused on reducing maternal and fetal morbidity and mortality; it focuses on steps taken now to protect the health of a baby in the future and consists of interventions aimed to minimize behavioral, social, and health-related risk to a woman’s (and a man’s) overall health and to improve outcomes for potential pregnancies. Pharmacists can help incorporate this care into their daily interactions with women of childbearing age. They are especially able to help women understand the potentials risks associated with medication use and assist them with various areas of preconception and prenatal care including immunizations, folic acid use, and management of chronic diseases.4,5
Medication Safety
The safety of medication in pregnancy involves the mother and the fetus. Pregnancy induces physiologic changes that affect the pharmacokinetics of medications, such as increased clearance, reduced half-life, and reduced area under the curve. In this vulnerable developmental state, certain medications can reach the fetus and cause harm either directly or indirectly. There are very little data available about optimal dosing and safety of medications in pregnant patients, mainly because involvement of pregnant women in clinical trials poses an ethical dilemma. Despite this, medication use during pregnancy is more common than not. More than 90% of women report taking at least one OTC or prescription medication during their pregnancy, with over 80% of women reporting using at least one medication during the first trimester. Medication use during pregnancy is often required for the optimal management of preexisting conditions such as hypertension or diabetes, or treatment of an acute condition such as a urinary tract infection, or for the management of a new condition resulting from the pregnancy, such as nausea and vomiting. Over the past four decades the average number of women using four or more OTC or prescription medications during pregnancy more than doubled, indicating an increasing trend of medication use in pregnancy.6-10
Timing of exposure to the fetus is a key determinant of risk. Immediate exposure of certain medications can result in spontaneous termination of a pregnancy, even before detection of the pregnancy. Many birth defects, including cleft lip or palate and neural-tube defects, occur within the first 60 days postconception. Pharmacists can provide a comprehensive preconception medication review for this patient population to help minimize such risks. The risks and benefits of continuing drug therapy (including supplements and herbals) during a possible pregnancy should be evaluated, and the pharmacist can recommend appropriate alternatives.
Teratogenic medications should be a focal point. The negative impact of these agents clearly outweighs any benefits. One study found that 25% of women of childbearing age were prescribed at least one high-risk medication, with over half not having evidence of contraception management. Some medications with known teratogenic risks include angiotensin-converting enzyme inhibitors, angiotensin-II receptor blockers, antiepileptic drugs, warfarin, tetracyclines, fluoroquinolones, trimethoprim, vitamin A, and vitamin A derivatives.4,11-13
When looking for information about medication and pregnancy, most women cited their physician, the product-information leaflet, and their pharmacist as the most-consulted sources. Pharmacists are in a prime position to help answer questions and alleviate concerns of pregnant patients. Pregnant women often either overestimate teratogenic risks of medications, which can worsen underlying conditions, or underestimate risks, with both scenarios leading to potential harm to the growing fetus.14,15
Classification of Drug Risk
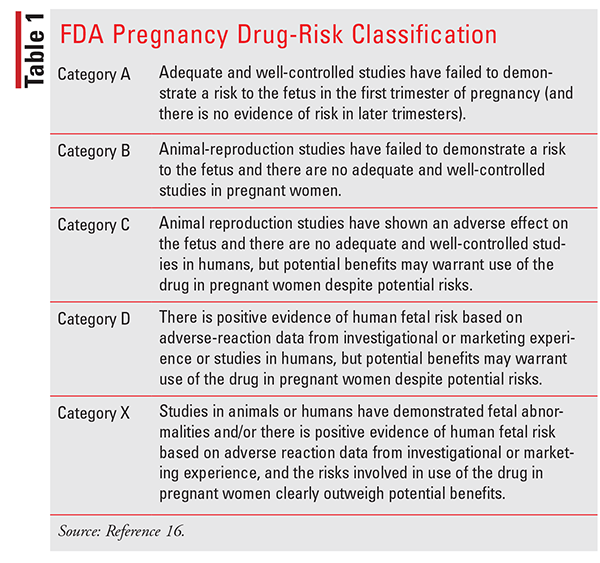
Healthcare providers have little information to help determine the potential risks and benefits to both mother and fetus due to the lack of randomized clinical trials in this patient population. To help assist with drug selection, the FDA introduced a drug-classification system, which has been in use since 1979 (TABLE 1). Although this classification provided a guideline for practitioners to follow to help determine the relative safety of medications, most of the data was derived from animal studies and uncontrolled studies in humans and did not accurately and consistently reflect the differences in degree of fetal risk. Practitioners relied too heavily on a system that was often misinterpreted and misused. To make a truly informed decision about medication use in pregnancy, more detailed information was needed. The FDA replaced this classification system in 2014 with a new system, Pregnancy and Lactation Labeling Rule (PLLR), with the intent of assisting practitioners in making evidence-based treatment decisions, minimizing misinformation. The current labeling provides information about the risks and benefits of medication use during pregnancy and lactation in a format that is consistent and includes data about use in both females and males of reproductive potential. Although this new system does provide more information regarding medication safety, it still requires an extensive risk-benefit analysis.16,17
Vitamins and Minerals
Significant metabolic changes occur throughout pregnancy that result from both intense fetal growth as well as altered maternal physiology. Such changes necessitate adequate maternal intake of nutrients, including vitamins and minerals, to both maintain maternal health and provide for adequate fetal growth and development. Deficiencies in nutritional intake during the gestational period have been associated with poor health outcomes for both the mother and developing fetus. As such, it is common practice to employ multivitamin supplementation during pregnancy, especially in those women who may not consume appropriate, healthy diets. In the U.S. certain women may be at increased risk for nutrient deficiencies, including adolescents, smokers, vegetarians, substance abusers, those who have had bariatric surgery, those with malabsorption syndromes, and those carrying more than one fetus. These women may benefit from a consultation with a dietician to ensure adequate micronutrient consumption.18-20
During pregnancy, adequate maternal iron intake is needed to maintain sufficient red blood cell production for an expanding plasma volume. Iron-deficiency anemia appears to occur in approximately 20% of patients and may be associated with adverse outcomes, including preterm labor, low birth weight, and impaired fetal brain development. Iron consumption of approximately 30 mg/day, an amount that is found in most prenatal vitamin supplements, is generally recommended to prevent iron-deficiency anemia. Pregnant women who are found to suffer from iron-deficiency anemia should receive further supplementation of 30 mg to 120 mg/day to correct their condition.18,21-24
Adequate maternal intake of folic acid is essential to prevent neural-tube defects in the developing fetus. It is generally recommended that women receive folic-acid supplements of 0.4 mg to 0.8 mg daily starting 1 month before conception to reduce the risk of neural-tube defects in the newborn. Most multivitamin prenatal supplements contain the recommended folic-acid requirement. Women at high risk of having a child with neural-tube defects may receive supplementation as high as 1 mg to 4 mg daily for the first 12 weeks of gestation.18,25-28
Calcium is important for proper fetal skeletal development as well as for maintaining and regulating maternal blood pressure during pregnancy. Although the daily recommended allowance of 1,000 mg of calcium for women does not differ based on pregnancy status, it is estimated that almost a quarter of all pregnant woman in the U.S. consume suboptimal quantities of this element. Supplementation with 1 gram to 2 grams of calcium daily is recommended for women with low baseline dietary calcium intake and high-risk pregnant patients as a means to minimize the risk of preeclampsia.18,29,30
Vitamin D deficiency is a common finding in pregnant women in the U.S., with an estimated prevalence of approximately 33%. Vitamin D deficiency has been associated with several negative health outcomes to both the mother and fetus including preeclampsia, gestational diabetes, postpartum depression, and low birth weight. Currently, the clear clinical benefit of routine vitamin D supplementation is controversial. However, the American College of Obstetricians and Gynecologists (ACOG) recommends routine supplementation of vitamin D with doses that are generally included in a prenatal vitamin (approximately 400 IU).18,31,32
Immunizations
The adherence to recommended vaccination schedules during pregnancy is essential to maintain adequate protection of both the mother and fetus against a variety of vaccine-preventable pathogens. Numerous agencies including the Advisory Committee on Immunization Practices (ACIP), the Infectious Diseases Society of America (IDSA), and ACOG have published general recommendations for the immunization of pregnant women. Ideally, women should receive all recommended vaccines prior to conception and in accordance with the CDC-recommended adult-immunization schedule. The immunization of women during pregnancy is generally recommended for susceptible patients when the risks to the mother and/or fetus are high and the administration of the vaccine is unlikely to cause harm. Immunizations that are recommended for all pregnant women as a routine part of prenatal care include the inactivated influenza vaccine (within season) and the adult formulation of the tetanus, diphtheria, acellular pertussis vaccine.33-36
ACIP recommends that all women who are or who might be pregnant during the influenza season receive the inactivated influenza vaccine as soon as it becomes available, and prior to the onset of influenza activity in their community. This recommendation is based on observations that pregnant and postpartum women are at a higher risk for severe illness and complications from influenza. Any licensed, recommended, and age-appropriate inactivated influenza vaccine can be administered regardless of the stage of pregnancy.36,37
In addition to the influenza vaccine, all pregnant women are recommended to receive the tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) routinely during each pregnancy. This recommendation is made both to protect the mother and to ensure the production and transmission of maternal antibodies to the newborn. Infants under 3 months of age are at greatest risk of morbidity and mortality from pertussis, with 69% of all reported pertussis deaths occurring in this age range. The transmission of maternal antibodies is necessary to protect infants until they begin receiving protection from their primary immunization series against tetanus, diphtheria, and pertussis. A single dose of Tdap vaccine is recommended by ACIP to be administered between 27 and 36 weeks of gestation to maximize maternal antibody production as well as antibody transfer to the fetus. Administration early in the recommended time frame is preferred as this dosing optimizes neonatal core blood pertussis-antibody levels and theoretically maximizes infant protection.38
Over the past year it has been shown that pregnant and postpartum women are at increased risk of developing severe symptoms from COVID-19 infections compared with nonpregnant patients. Pregnant patients are more likely to require hospitalization, and ICU stay, and they have a higher mortality rate compared with controls. In addition, infection with COVID-19 during pregnancy has been associated with adverse effects on the fetus, including an increased risk of preterm birth. For these reasons, the CDC recommends that pregnant patients consider receiving recommended COVID-19 vaccines according to current guidance documents. At press time, neither the CDC nor the FDA has identified any maternal or fetal safety concerns when the currently available COVID-19 vaccines have been administered in pregnancy.39
Despite these recommendations, maternal vaccination rates with influenza vaccine and Tdap remain disappointingly low. A recent survey by the CDC suggests that during the 2019-2020 season, only 61.2% and 56.6% of pregnant women received the influenza vaccine and Tdap, respectively. This illustrates the need for pharmacists to take an active role in promoting the appropriate use of vaccines for their patients who are, or are considering, becoming pregnant.36
Sources of Information
To fully provide evidence-based recommendations, pharmacists need to be aware of the available resources relevant to pregnancy and lactation. The PLLR that has replaced the FDA’s original classification system provides summaries of the risks associated with prescription medications and biologic products approved after June 30, 2001. This narrative also provides data supporting the summaries and is required to be updated when the information is outdated. In addition, there is a clinical-consideration section that addresses risk assessment and how to handle inadvertent fetal exposure. It is important to also consult the primary literature, including case reports, case-control studies, prospective cohort studies, and historical cohort studies. Other resources include teratology information services which provide pregnant women with the potential risks of medication use and follow them throughout the pregnancy to assess the outcomes, pregnancy registries, and computerized databases. Useful resources are provided in TABLE 2.40

Conclusion
Many pregnancies are unplanned. It is important to begin providing preconception care to all women of child-bearing age regardless of a woman’s intention to become pregnant. The delivery of preconception care can easily be incorporated as part of medication-therapy management. A medication review can be conducted to ensure optimal management of chronic conditions and to identify medications that may potentially pose a risk to the fetus if a pregnancy should occur. The pharmacist, healthcare provider, and patient can consider the risks and benefits associated with continued medication use and/or discuss the possibility of a safer but just as efficacious alternative. Medication selection should be based on current evidence. A thorough review also provides the opportunity to ensure adequate folic acid, vitamin D, and calcium intake as well as ensuring recommended vaccinations are provided.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.

Why do I need prenatal care?
Prenatal care is one of the best ways to keep you and your baby safe during pregnancy. Without prenatal care, your baby is at risk for complications such as low birth weight, premature birth, and death. However, the best care you can give to your unborn baby is to take care of yourself before pregnancy. This is called preconception care.
What is preconception care?
Preconception care has become very important in reducing risk factors that can complicate a pregnancy and cause harm to your unborn baby. This type of care is important for all women, not just those who are planning to become pregnant. It consists of health education, health promotion, and screenings and interventions to help women live a healthier life. It means taking control and choosing healthy habits. Preconception health means babies are less likely to have low birth weight or be born too early. They are also less likely to be born with birth defects or other disabling conditions.
What are some things that I can do now?
If you have a chronic health condition such as high blood pressure, diabetes, depression, or obesity, you want to work with your team of healthcare providers, such as your doctor and pharmacist, to keep your condition well controlled. The more controlled these conditions are before your pregnancy, the fewer complications you and your baby will have. Medications should be continued to help control your conditions. You should review your medications with your pharmacist to help identify any medications that may be potentially harmful if you become pregnant. Your pharmacist and doctor will then work together to switch you to other agents that are just as effective but are considered safer in case a pregnancy happens. You should also take 400 to 800 micrograms of folic acid every day. This amount can be found in a daily multivitamin along with calcium, iron, and vitamin D. It is also important to have a well-balanced and nutritionally sound diet. If you currently smoke or drink, you should stop. It also very important to be up-to-date with all required vaccinations. You want to be sure you receive your flu vaccine every year.
Where can I find more information?
The CDC: Before Pregnancy: www.cdc.gov/preconception/index.html.
March of Dimes: www.marchofdimes.org/
pregnancy/your-checkup-before-pregnancy.aspx.
U.S. Dept. of Health & Human Services: Office on Women’s Health: www.womenshealth.gov/pregnancy.
REFERENCES
1. CDC. Reproductive health: infant mortality. September 10, 2020. www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm. Accessed June 13, 2021.
2. Finer LB, Zolna MR. Declines in unintended pregnancy in the United States, 2008-2011. N Engl J Med. 2016;374(9):843-852.
3. Johnson K, Posner SF, Biermann J, et al. Recommendations to improve preconception health and health care--United States. A report of the CDC/ATSDR Preconception Care Work Group and the Select Panel on Preconception Care. MMWR Recomm Rep. 2006;55(RR-6):1-23.
4. Wilkes J. AAFP releases position paper on preconception care. Am Fam Physician. 2016;94(6):508-510.
5. CDC. Pregnancy overview: preconception care. Published August 29, 2014. www.cdc.gov/preconception/overview.html. Accessed June 13, 2021.
6. Hamilton B, Martin J, Osterman M. Births: provisional data for 2020. National Center for Health Statistics; 2021. cdc.gov/nchs/data/vsrr/vsrr012-508.
7. Pariente G, Leibson T, Carls A, et al. Pregnancy-associated changes in pharmacokinetics: a systematic review. PLoS Med. 2016;13(11):e1002160.
8. Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512-519.
9. Ross EJ, Graham DL, Money KM, Stanwood GD. Developmental consequences of fetal exposure to drugs: what we know and what we still must learn. Neuropsychopharmacology. 2015;40(1):61-87.
10. Mitchell AA, Gilboa SM, Werler MM, et al. Medication use during pregnancy, with particular focus on prescription drugs: 1976-2008. Am J Obstet Gynecol. 2011;205(1):51.e1-8.
11. Dunlop AL, Gardiner PM, Shellhaas CS, et al. The clinical content of preconception care: the use of medications and supplements among women of reproductive age. Am J Obstet Gynecol. 2008;199(6 Suppl 2):S367-S372.
12. Panchal BD, Cash R, Moreno C, et al. High-risk medication prescriptions in primary care for women without documented contraception. J Am Board Fam Med. 2019;32(4):474-480.
13. Tsamantioti ES, Hashmi MF. Teratogenic medications. In: StatPearls. StatPearls Publishing; 2021. www.ncbi.nlm.nih.gov/books/NBK553086/. Accessed June 13, 2021.
14. Nordeng H, Ystrøm E, Einarson A. Perception of risk regarding the use of medications and other exposures during pregnancy. Eur J Clin Pharmacol. 2010;66(2):207-214.
15. Damase-Michel C, Christaud J, Berrebi A, et al. What do pregnant women know about non-steroidal anti-inflammatory drugs? Pharmacoepidemiol Drug Saf. 2009;18(11):1034-1038.
16. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Federal Register. Published May 29, 2008. www.federalregister.gov/documents/2008/05/29/E8-11806/content-and-format-of-labeling-for-human-prescription-drug-and-biological-products-requirements-for. Accessed June 10, 2021.
17. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Federal Register. Published December 4, 2014. www.federalregister.gov/documents/2014/12/04/2014-28241/content-and-format-of-labeling-for-human-prescription-drug-and-biological-products-requirements-for. Accessed June 10, 2021.
18. Brown B, Wright C. Safety and efficacy of supplements in pregnancy. Nutr Rev. 2020;78(10):813-826.
19. Moos M-K, Dunlop AL, Jack BW, et al. Healthier women, healthier reproductive outcomes: recommendations for the routine care of all women of reproductive age. Am J Obstet Gynecol. 2008;199(6 Suppl 2):S280-S289.
20. Sullivan KM, Ford ES, Azrak MF, Mokdad AH. Multivitamin use in pregnant and nonpregnant women: results from the Behavioral Risk Factor Surveillance System. Public Health Rep. 1974. 2009;124(3):384-390.
21. Figueiredo ACMG, Gomes-Filho IS, Silva RB, et al. Maternal anemia and low birth weight: a systematic review and meta-analysis. Nutrients. 2018;10(5):601.
22. Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Am J Clin Nutr. 2011;93(6):1312-1320.
23. Cantor AG, Bougatsos C, Dana T, et al. Routine iron supplementation and screening for iron deficiency anemia in pregnancy: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162(8):566-576.
24. Institute of Medicine (US) Committee on the Prevention, Detection, and Management of Iron Deficiency Anemia Among U.S. Children and Women of Childbearing Age. Iron deficiency anemia: recommended guidelines for the prevention, detection, and management among U.S. children and women of childbearing age. Earl R, Woteki CE, eds. National Academies Press (US); 1993. Accessed June 13, 2021.
25. Crider KS, Bailey LB, Berry RJ. Folic acid food fortification—its history, effect, concerns, and future directions. Nutrients. 2011;3(3):370-384.
26. De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, Rayco-Solon P. Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev. 2015;(12):CD007950.
27. U.S. Preventive Services Task Force. Folic acid for the prevention of neural tube defects: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150(9):626-631.
28. Arth A, Tinker S, Moore C, et al. Supplement use and other characteristics among pregnant women with a previous pregnancy affected by a neural tube defect—United States, 1997-2009. MMWR Morb Mortal Wkly Rep. 2015;64(1):6-9.
29. Guideline: Calcium supplementation in pregnant women. World Health Organization; 2013. www.ncbi.nlm.nih.gov/books/nbk154180/. Accessed June 13, 2021.
30. Millen BE, Abrams S, Adams-Campbell L, et al. The 2015 Dietary Guidelines Advisory Committee Scientific Report: development and major conclusions. Adv Nutr. 2016;7(3):438-444.
31. Palacios C, Gonzalez L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol. 2014;144 Pt A:138-145.
32. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 495: Vitamin D: screening and supplementation during pregnancy. Obstet Gynecol. 2011;118(1):197-198.
33. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 741: maternal immunization. Obstet Gynecol. 2018;131(6):e214-e217.
34. Pickering LK, Baker CJ, Freed GL, et al. Immunization programs for infants, children, adolescents, and adults: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49(6):817-840.
35. CDC. ACIP vaccine recommendations and schedules. Published March 4, 2021. www.cdc.gov/vaccines/acip/recommendations.html. Accessed June 13, 2021.
36. Razzaghi H, Kahn K, Black CL. Influenza and Tdap vaccination coverage among pregnant women—United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1391-1397.
37. Grohskopf LA, Alyanak E, Broder KR. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices—United States, 2020–21 Influenza Season. MMWR Recomm Rep. 2020;69:1-24.
38. Havers FP, Moro PL, Hunter P, et al. Use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines: updated recommendations of the Advisory Committee on Immunization Practices—United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(3):77-83.
39. CDC. COVID-19 Vaccines while pregnant or breastfeeding. Published June 29, 2021. www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html. Accessed June 30, 2021.
40. Feibus KB. FDA’s proposed rule for pregnancy and lactation labeling: improving maternal child health through well-informed medicine use. J Med Toxicol. 2008;4(4):284-288.
To comment on this article, contact rdavidson@uspharmacist.com.






