US Pharm. 2018:12(43):36-37.
Cytomegalovirus (CMV) is a member of the herpesvirus family. CMV is a common virus that is usually harmless to people with healthy immune systems. After an initial infection, the virus remains latent in the body and can reactivate at any time.1 For those with a normal immune system, infection with the virus is usually asymptomatic. When symptoms occur, they may appear cold-like; the virus may cause mononucleosis or hepatitis.1 The CMV may cause serious issues in those with a compromised immune system and can cause a congenital CMV infection in infants.2
Epidemiology and Transmission
According to the CDC, nearly one in three children is infected with CMV by the age of 5 years, and over half of adults have been infected by the age of 40 years, with most being asymptomatic.³
Transmission of the virus is similar to that of the other herpesviruses—by contact with bodily fluids. It can be transmitted sexually, during birth, through breast milk, through contact with saliva, or through organ transplants and blood infusions.³
Diagnosis
Diagnosis is confirmed when CMV antibodies are detected through serology tests. The most common test used is the enzyme-linked immunosorbent assay (ELISA).4 ELISA is used for individuals aged 12 months and older; a positive CMV immunoglobulin G (IgG) indicates infection. Initially, CMV IgG is measured in two samples taken 1 to 3 months apart and is used to diagnose primary infection.4 CMV immunoglobulin M is present in secondary infections and cannot be used to diagnose a primary infection.4
Congenital CMV in newborns is diagnosed using saliva. The technique used to diagnose CMV is called polymerase chain reaction (PCR) assay.4 False-positives can occur because the CMV virus from the mother’s breast milk can remain in the infant’s saliva shortly after being breast-fed. Because of this, urine is tested for confirmation.4
Treatment
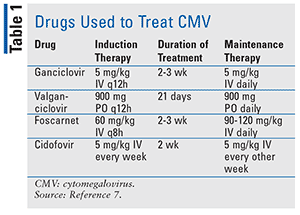
Mild CMV infections do not require treatment and usually subside on their own. Treatment with antivirals is reserved for times when the infection threatens life or eyesight. Congenital and perinatal CMV consists of supportive therapy along with either parenteral ganciclovir or oral valganciclovir 16 mg/kg twice a day for 6 months.5 These treatments prevent hearing deterioration, improve developmental outcomes, and decrease viral shedding.5 Drug names and dosages of the antivirals that are used to treat CMV can be found in Table 1.
Note that ganciclovir has a black box warning for anemia, thrombocytopenia, and granulocytopenia. Valganciclovir carries a black box warning for severe leukopenia, neutropenia, anemia, and thrombocytopenia, and may impair fertility. IV foscarnet has a black box warning for renal impairment and seizures related to alteration in plasma minerals and electrolytes. Cidofovir has a black box warning for acute renal failure resulting in dialysis or death.6 Consider stopping treatment if CD4 counts are greater than or equal to 100 cells/µL for 3 months.7
RealTime CMV Assay
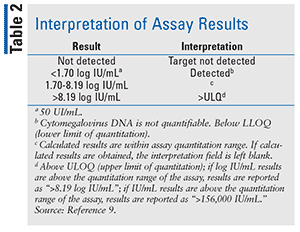
Designed by Abbott Molecular Inc., the RealTime CMV assay (Figure) uses in vitro PCR assays to test for CMV DNA in humans. It is used to aid in the management of anti-CMV therapy in hematopoietic stem cell transplant patients.8 The CMV assay must be interpreted in clinical laboratory findings. It is not used to screen for CMV DNA in the blood. The use of the RealTime CMV assay is limited because it can only be used in individuals who have undergone a hematopoietic stem cell transplant.8 It has not been tested on patients in any other category (such as other transplant procedure, neonates, pediatric patients, or AIDS patients) or in patients who are in an immunocompromised state. Detection of CMV DNA is contingent upon proper collection, handling, transportation, storage, and preparation of the specimen. A false-negative can result from improper conditions of the specimen.8 The assay amplifies two selected targets of the CMV genome. This is done in order to reduce the risk of rare mutations that could result in the assay failing to detect the virus.8 Table 2 indicates how to interpret the results of the assay. Results are reported as either IU/mL or log IU/mL.9
Efficacy
Several studies have demonstrated the efficacy of the RealTime CMV assay. In a 2015 study by Tremblay and colleagues, the RealTime CMV assay was compared with the Roche Cobas Amplicor CMV Monitor Test. The concordance of the two tests was 98%, and it was concluded that the RealTime CMV assay had good sensitivity and precision and is suitable for monitoring the CMV in transplant patients.10 An earlier study by Schnepf and colleagues compared the RealTime CMV assay to the Qiagen artus CMV assay. This study found that the RealTime CMV assay showed higher sensitivity (42.7%) when compared to the Qiagen artus CMV assay (35.4%).11 It also found that the RealTime CMV assay had a 47.8% increase in quantified samples (32.8%) as opposed to the Qiagen artus CMV assay (22.2%).11 The study concluded that the RealTime CMV assay had higher sensitivity and would allow more precise assessment of the kinetics of viral replication for early detection in certain individuals.11
Conclusion
The RealTime CMV assay has demonstrated effectiveness for use in clinical and laboratory evaluations. Although currently it is only used in the management of patients with hematopoietic stem cell transplants, it is becoming a routine test in the laboratory. More information can be found at www.molecular.abbott/us/en/products/infectious-disease/realtime-cmv.
REFERENCES
1. Friel T. Epidemiology, clinical manifestations, and treatment of cytomegalovirus infection in immunocompetent adults. August 25, 2017. UpToDate. www.uptodate.com/contents/epidemiology-clinical-manifestations-and-treatment-of-cytomegalovirus-infection-in-immunocompetent-adults. Accessed September 1, 2018.
2. National CMV Foundation. What is CMV? www.nationalcmv.org/overview/start-here.aspx. Accessed September 1, 2018.
3. CDC. About CMV. www.cdc.gov/cmv/overview.html. Accessed September 2, 2018.
4. CDC. Laboratory diagnosis of CMV infection for persons 12 months of age. www.cdc.gov/cmv/clinical/lab-tests.html. Accessed September 2, 2018.
5. Tesini BL. Congenital and perinatal cytomegalovirus infection (CMV). Merck Manual. Kenilworth, NJ: Merck Sharp & Dohme Corp; 2018. www.merckmanuals.com/professional/pediatrics/infections-in-neonates/congenital-and-perinatal-cytomegalovirus-infection-cmv. Accessed September 2,2018.
6. IBM Micromedex Drugdex. Greenwood Village, CO: IBM Watson Health. www.micromedexsolutions.com. Accessed November 26, 2018.
7. Kaye KM. Cytomegalovirus (CMV) infection. Merck Manual. Kenilworth, NJ: Merck Sharp & Dohme Corp; 2018. www.merckmanuals.com/professional/infectious-diseases/herpesviruses/cytomegalovirus-cmv-infection. Accessed September 2, 2018.
8. Abbott. Products. Abbott RealTime CMV. www.molecular.abbott/us/en/products/infectious-disease/realtime-cmv. Accessed September 2, 2018.
9. Abbott RealTime CMV Assay package insert. Des Plaines, IL: Abbott Molecular Inc. 2017.
10. Tremblay MA, Rodrigue MA, Deschenes L, et al. Cytomegalovirus quantification in plasma with Abbott RealTime CMV and Roche Cobas Amplicor CMV assays. J Virol. Methods. 2015;225:1-3.
11. Schnepf N, Scieux C, Resche-Riggon M, et al. Fully automated quantification of cytomegalovirus (CMV) in whole blood with the new sensitive Abbott realtime CMV assay in the era of the CMV international standard. J Clin Microbiol. 2013;51(7):2096-2102.
To comment on this article, contact rdavidson@uspharmacist.com.