US Pharm. 2024;49(7):4-8.
Respiratory syncytial virus (RSV) has long been identified as a leading cause of acute respiratory illnesses in young children. In the United States, it results in an estimated 58,000 to 80,000 hospitalizations annually among children aged younger than 5 years, with infants being particularly vulnerable; however, RSV is not confined to pediatric populations. RSV increasingly affects older adults, causing an estimated 60,000 to 160,000 hospitalizations and 6,000 to 10,000 deaths annually among adults aged 65 years or older. RSV also imposes a significant economic burden on adults, especially those aged 50 years and older. A recent study estimated that among U.S. adults aged 60 years and older, RSV cases contribute to an annual economic burden of $6.6 billion, encompassing both direct medical costs (such as hospitalization expenses) and indirect costs (such as lost productivity).1-3
RSV is a highly contagious, enveloped RNA virus primarily affecting the respiratory tract. While RSV typically causes mild, cold-like symptoms in most healthy individuals, it can lead to severe lower respiratory tract infections (e.g., bronchiolitis and pneumonia) in high-risk groups. These groups include infants, young children, older adults, and individuals with compromised immune systems or underlying respiratory conditions. The virus spreads through respiratory droplets, direct contact with contaminated surfaces, and close personal contact. It can survive for up to 6 hours on nonporous hard surfaces such as countertops, glass, plastics, and metals, up to 30 minutes on the skin, and up to 2 hours on porous surfaces, such as paper and cardboard.4
Following an incubation period of approximately 4 to 6 days, RSV begins replicating in the nasopharyngeal epithelium and subsequently spreads to the lower respiratory tract, although the precise mechanism remains unclear. Infected individuals can transmit the virus from a few days before symptom onset until 1 to 2 weeks after recovery, with immunocompromised patients potentially shedding the viable virus for even longer periods.4
Preventive strategies include practicing good hand hygiene, avoiding close contact with infected individuals, and ensuring proper ventilation. While RSV is generally self-limiting in healthy individuals, severe cases may require supportive care, including supplemental oxygen and respiratory support. Until recently, only palivizumab, a monoclonal antibody (mAb) requiring monthly dosing, was available for RSV prevention in eligible infants and young children with specific underlying conditions. However, in 2023, newly licensed and recommended immunizations marked a historic turning point in RSV prevention for both young children and older adults, offering new hope for vulnerable populations.
Pharmacists play a pivotal role in vaccine administration, education, and patient counseling. Staying informed about RSV and keeping up to date with the latest research and guidelines enable pharmacists to provide evidence-based recommendations and optimize patient outcomes, contributing to the control of RSV spread.
Risk Factors
RSV poses significant risks, particularly to certain vulnerable populations; age is a notable risk factor. Infants, especially those aged younger than 6 months, are at heightened risk due to their still-developing immune system and smaller airways, which are more susceptible to obstruction from inflammation and mucus. Premature infants (less than 32 weeks’ gestation) and those with congenital heart or chronic lung disease face even greater risks of severe RSV infection. Similarly, adults aged older than 65 years, especially those with chronic heart disease (CHD), chronic obstructive pulmonary disease (COPD), or immunocompromising conditions, are more likely to experience severe outcomes from RSV. Other at-risk individuals, regardless of age, include those with a compromised immune system and underlying chronic conditions, such as asthma or COPD. Environmental factors also play a role; crowded living conditions and exposure to tobacco smoke can increase the likelihood of RSV transmission and severity.1,2,4
Long-term care facilities (LTCFs), including nursing homes, present a heightened risk for residents to contract and develop severe RSV infections. Studies have shown that the risk of severe RSV infection is higher among older adults in LTCF settings compared with adults in community-dwelling settings. LTCF residents are typically older adults (>65 years) who often have underlying chronic health conditions like CHD, COPD, or weakened immune systems. These conditions significantly increase the risk of serious complications from RSV. The close living quarters, shared spaces, and frequent interactions in LTCFs contribute to increased RSV transmission risk.2,5,6
Understanding these risk factors is crucial for pharmacists to identify at-risk patients and recommend preventive measures to reduce the spread of RSV.
Clinical Presentation and Diagnosis
RSV infection manifests differently depending on the age and underlying health of the infected individual. Symptoms typically develop 4 to 7 days after exposure, but individuals are most contagious 1 to 2 days before symptoms appear. In otherwise healthy children, RSV usually starts as an upper respiratory illness (URI) with a gradual onset. Common initial symptoms include a runny nose, congestion, cough, and low-grade fever, which can progress to wheezing and difficulty breathing. This is especially true for infants, whose narrower airways are more prone to obstruction by inflammation and mucus. In some cases, young infants may experience apnea due to RSV infection, especially premature infants or those with underlying conditions.4,7
Adults with RSV infection usually have mild or no symptoms, typically presenting with URI symptoms that last about 5 days. High-risk individuals, such as those with chronic heart or lung disease, may experience a more severe lower respiratory illness characterized by fever, cough, shortness of breath, and wheezing. In these populations, RSV can exacerbate underlying conditions and lead to complications such as pneumonia, bronchitis, and even respiratory failure requiring hospitalization.4,7
Diagnosing an RSV infection involves considering clinical presentation, risk factors, and laboratory testing. During the RSV season, clinicians should be vigilant for RSV in patients with acute respiratory symptoms, especially patients who are at high risk. Clinical history and physical examination findings, such as fever, cough, wheezing, respiratory distress, and auscultatory abnormalities, can provide valuable clues. However, laboratory confirmation is essential for a definitive diagnosis. Rapid antigen detection tests, which identify RSV antigens from nasopharyngeal swabs or aspirates, are widely used due to their ease of use and relatively high specificity, being up to 90% accurate in children and infants. Despite this, they have lower sensitivity compared with molecular tests such as the highly sensitive reverse transcription-polymerase chain reaction assays, which are the gold standard for RSV diagnosis. They offer superior sensitivity and the ability to differentiate between RSV subtypes (A and B). These molecular tests are generally used in older children, adults, and immunocompromised populations.7
Prompt and accurate diagnosis of RSV is crucial for implementing appropriate infection-control measures, initiating timely antiviral or immunomodulatory therapy (if indicated), and guiding supportive care, particularly in high-risk populations.
Management
In recent years, the unprecedented surge in RSV cases has underscored the importance of supportive care and symptom management, along with efforts to curb transmission. Typically, RSV infections are self-limiting, and the primary approach is to manage symptoms. However, the clinical management strategies for RSV vary between adults and children. Most infected infants and children are effectively managed as outpatients. In contrast, elderly people and immunocompromised individuals, especially those with underlying conditions that exacerbate RSV symptoms, are more likely to require hospitalization. Premature infants are also at a higher risk of needing hospital care. Currently, there are few antiviral treatment options available, but antiviral therapy is more commonly considered and administered in cases involving lung transplant recipients, immunocompromised individuals, and those who have undergone hematopoietic stem cell transplantation. For severe cases in pediatrics, antiviral drugs such as aerosolized ribavirin are recommended. As with other viral infections, the management of RSV primarily involves supportive care, with antiviral treatments reserved for more severe instances.4
Prevention
While there is no specific cure for RSV, various preventive strategies can effectively lower the risk of infection in both infants and adults. It is particularly important to prevent the spread of RSV among susceptible groups, such as infants and adults with a compromised immune system. To protect infants, the emphasis is on minimizing their exposure to the virus. This involves maintaining good hand hygiene by regularly washing hands with soap and water, especially before touching the infant, and using alcohol-based hand sanitizers when washing is not possible. Caregivers and family members should steer clear of close contact with the infant if they are feeling unwell and should cover their mouth and nose with tissues or their elbow when coughing or sneezing.
For infants at a greater risk of severe RSV outcomes, such as those born prematurely or those with certain heart or lung conditions, monthly injections of palivizumab, an mAb, are recommended during the RSV season. This provides passive immunity and lowers the risk of severe illness. This preventive treatment is typically given from late fall to early spring, when RSV is most prevalent.8
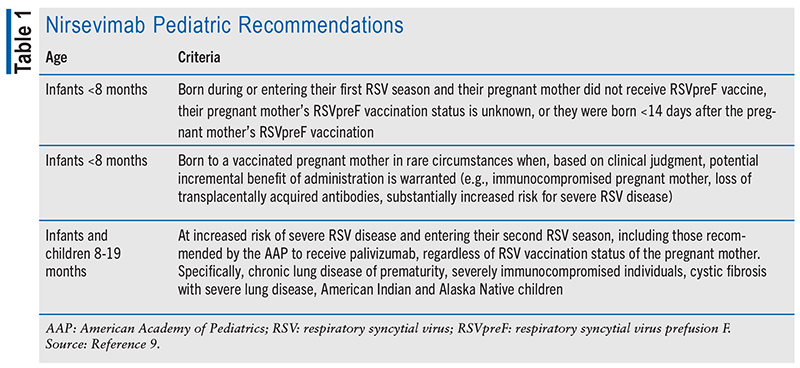
In July 2023, the FDA approved nirsevimab, a long-acting mAb, for the prevention of lower respiratory tract disease (LRTD) caused by RSV in infants. Nirsevimab is preferred over palivizumab due to its higher efficacy, longer-lasting effects, and simpler administration. The American Academy of Pediatrics, in agreement with the Advisory Committee on Immunization Practices (ACIP), recommends nirsevimab for infants aged younger than 8 months who are experiencing or entering their first RSV season, as well as for infants and children aged between 8 and 19 months who are at a higher risk of severe RSV disease and are entering their second RSV season (see TABLE 1). Nirsevimab should be administered just before the start of the RSV season. In the continental U.S., the optimal timing is from October to the end of March. If an infant is born just before or during the RSV season, nirsevimab should be given within 1 week of birth; however, nirsevimab can be administered to eligible infants and children at any time during the season if they have not yet received a dose.1,9
In May 2023, the FDA approved the first two vaccines for preventing RSV LRTD in adults aged 60 years and older: RSVpreF (Abrysvo) and RSVpreF3 (Arexvy). Both approved RSV vaccines are single-dose, recombinant stabilized prefusion F protein (preF) vaccines that are given by IM injection. Additionally, studies indicate that both vaccines offer protection against RSVA and RSVB. A key difference between the two vaccines is that RSVpreF3 is adjuvanted, while RSVpreF is not. Studies have shown that both vaccines provide moderate protection against RSV illness in older adults, with efficacy data suggesting a reduction of 60% to 80% in medically attended RSV LRTD and a similar decrease in severe RSV illness requiring hospitalization. Although not perfect, this level of protection can substantially lessen the impact of RSV on healthcare systems during peak seasons. Clinical trials have demonstrated that both vaccines are generally well tolerated, with mild side effects reported in a small percentage of recipients. These side effects were typically transient and included injection-site pain, fatigue, headache, and muscle aches.2
The ACIP recommends that adults aged 60 years or older may receive a single dose of RSV vaccine using shared clinical decision-making. This approach differs from other routine age-based and risk-based vaccine recommendations wherein the default is to vaccinate all individuals in a specified age range or risk category. Shared clinical decision-making involves an open discussion between healthcare providers and patients to determine if the benefits of RSV vaccination outweigh the risks on a case-by-case basis. This allows for flexibility based on what is best for each patient, considering factors such as the patient’s health status, risk of severe RSV disease, the provider’s clinical judgment, patient preferences, and the safety profile of the RSV vaccine products.2,7
In August 2023, the FDA approved RSVpreF for use in pregnant women. In September 2023, the ACIP recommended a single dose of the vaccine for pregnant women between 32 and 36 weeks’ gestation. Similarly, the American College of Obstetricians and Gynecologists recommends a single dose of this vaccine for pregnant women between 32 and 36 weeks’ gestation during the RSV season. The vaccine protects newborns and infants from severe RSV disease in the first 6 months after birth. Vaccinating pregnant women in the second or third trimester can help protect babies when they are most susceptible to developing severe illness.10
Role of the Pharmacist
As highly accessible healthcare professionals, pharmacists are instrumental in combating RSV. They can identify individuals at a higher risk of developing severe RSV complications by assessing medical histories and medication use. This includes young infants, particularly those aged younger than 6 months, premature babies, and those with chronic lung or heart conditions, as well as adults aged older than 65 years, especially those with CHD, COPD, or a compromised immune system. Pharmacists offer education to patients, caregivers, and the general public about RSV, covering transmission methods, symptoms, and preventive actions such as handwashing and avoiding contact with infected individuals. Crucially, pharmacists are pivotal in raising awareness about available vaccines and addressing any doubts patients may have about their effectiveness or safety. Currently, approximately 24.4% of adults aged 60 years and older report having received an RSV vaccine. Pharmacists can significantly contribute to increasing this figure by keeping abreast of the latest RSV vaccine guidelines for adults aged 65 years and older and pregnant women and by working closely with other healthcare professionals to ensure a thorough approach to RSV prevention.11
Conclusion
RSV remains a considerable challenge to public health, especially impacting infants, the elderly, and individuals with preexisting health issues. Identifying risk factors early, swift diagnosis based on clinical symptoms and laboratory tests, and the immediate application of suitable management techniques are essential in reducing severe illness and potential complications. The recent approval of RSV vaccines for older adults, along with the endorsement of vaccinating pregnant women to passively immunize newborns, marks a significant advancement in RSV prevention strategies. Pharmacists, as readily available healthcare providers, are well placed to inform patients, raise awareness, suggest appropriate vaccinations, and advise on the responsible use of OTC and prescription medications for managing RSV infections. By adopting a comprehensive and multifaceted approach that includes prevention, early detection, and optimal treatment, the substantial health impact of RSV can be effectively managed, leading to improved patient outcomes and a reduction in RSV-related illness and death across all age groups.

What Is RSV?
Respiratory syncytial virus (RSV) is a common respiratory virus that infects the lungs and airways. It is a leading cause of illness in young children but can affect adults as well. RSV season typically occurs during fall, winter, and spring.
How Does RSV Spread?
RSV is highly contagious. The virus spreads easily through coughing, sneezing, and contact with respiratory droplets from an infected person or with contaminated surfaces or objects. The virus can live on surfaces for a while. Touching a contaminated surface and then your face can introduce the virus. Infected individuals are typically contagious 1 to 2 days before symptoms appear and remain so for 3 to 8 days after symptoms begin.
What Are the Symptoms of RSV?
In infants and young children, RSV can lead to bronchiolitis (inflammation of the small airways) and pneumonia. Common symptoms include a runny nose, cough, sneezing, fever, wheezing, rapid breathing, or difficulty breathing. In most healthy adults and older children, RSV symptoms are typically mild, resembling a cold. However, RSV can cause more serious illness in the elderly and adults with chronic heart or lung disease or a weakened immune system.
Who Is at Risk for Severe RSV Infection?
Not everyone gets a bad infection from RSV. However, RSV can lead to serious lung infection, breathing problems, and hospitalization for some people who are at higher risk. These include infants who are aged younger than 6 months, especially premature babies or those with chronic health conditions. Adults aged 65 years and older are also at risk, especially if they have health conditions such as chronic obstructive pulmonary disease, chronic heart disease, or a weakened immune system.
How Do You Treat RSV?
There is no specific treatment for RSV infections, but some medications may be used to help manage symptoms such as fever and congestion. Since RSV is a virus, antibiotics are not effective against it. Most RSV infections go away on their own within 1 to 2 weeks in healthy children and adults. Hospitalization may be required for infants or those with severe symptoms to provide oxygen therapy and IV fluids.
How Can I Prevent RSV?
Wash your hands often with soap and water for at least 20 seconds, especially before and after being around someone who is sick, before eating, and after changing a diaper. Avoid close contact with people who are sick with a cold or experiencing RSV symptoms. Cover your cough or sneeze with a tissue or your elbow. Throw used tissues away in the trash immediately. Regularly clean and disinfect surfaces that are frequently touched, especially doorknobs, countertops, and toys. Adults aged 65 years and older can get an RSV vaccine to help reduce the risk of severe illness. Pregnant women can get an RSV vaccine during their pregnancy to help protect their newborn from RSV during the first few months of life.
Where Can I Go to Learn More?
CDC: www.cdc.gov/rsv
RSV and Me: www.rsvandme.com
REFERENCES
1. Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(34):920-925.
2. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793-801.
3. Carrico J, Hicks KA, Wilson E, et al. The annual economic burden of respiratory syncytial virus in adults in the United States. J Infect Dis. 2023:jiad559.
4. Kaler J, Hussain A, Patel K, et al. Respiratory syncytial virus: a comprehensive review of transmission, pathophysiology, and manifestation. Cureus. 2023;15(3):e36342.
5. Bosco E, van Aalst R, McConeghy KW, et al. Estimated cardiorespiratory hospitalizations attributable to influenza and respiratory syncytial virus among long-term care facility residents. JAMA Netw Open. 2021;4(6):e2111806.
6. Narejos Pérez S, Ramón Torrell JM, Põder A, et al. Respiratory syncytial virus disease burden in community-dwelling and long-term care facility older adults in Europe and the United States: a prospective study. Open Forum Infect Dis. 2023;10(4):ofad111.
7. CDC. RSV information for healthcare providers. March 8, 2024. www.cdc.gov/rsv/clinical/index.html. Accessed June 3, 2024.
8. Zhang XL, Zhang X, Hua W, et al. Expert consensus on the diagnosis, treatment, and prevention of respiratory syncytial virus infections in children. World J Pediatr. 2024;20(1):11-25.
9. American Academy of Pediatrics. AAP recommendations for the prevention of RSV disease in infants and children. February 21, 2024. www.publications.aap.org/redbook/resources/25379/AAP-Recommendations-for-the-Prevention-of-RSV. Accessed June 4, 2024.
10. American College of Obstetricians and Gynecologists. Maternal respiratory syncytial virus vaccination. www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2023/09/maternal-respiratory-syncytial-virus-vaccination. Accessed June 4, 2024.
11. CDC. RSVVaxView. May 21, 2024. www.cdc.gov/vaccines/imz-managers/coverage/rsvvaxview/index.html. Accessed June 4, 2024.
The content contained in this article is for informational purposes only. The content is not intended to be a substitute for professional advice. Reliance on any information provided in this article is solely at your own risk.
To comment on this article, contact rdavidson@uspharmacist.com.





