US Pharm. 2018;43(5)(Specialty&Oncology suppl):12-15.
ABSTRACT: Factor V Leiden thrombophilia is a genetic disorder that may increase a patient’s risk of developing a venous thromboembolism (VTE). Current management strategies involve the use of pharmacotherapy, when indicated, in the event of deep venous thrombosis or pulmonary embolism. Treatment guidelines have specific recommendations regarding the use of oral anticoagulant therapy for the treatment and prevention of VTE events. Pharmacotherapy options include vitamin K antagonists or direct oral anticoagulants, such as direct thrombin inhibitors, and factor Xa inhibitors. Owing to multiple considerations concerning site and cause of the VTE, it can be daunting to select an agent. Understanding the therapy options for factor V Leiden will be beneficial for pharmacists who have the opportunity to answer questions and provide patient counseling about this disease.
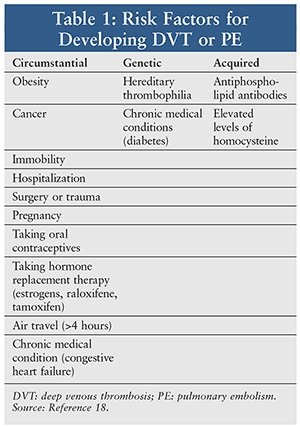
Venous thromboembolism (VTE) is a major medical problem that affects between 1 and 2 in 1,000 persons per year.1 A variety of genetic, acquired, and circumstantial risk factors contribute to the development of VTE (Table 1). In patients who present with VTE, one-half are identified as having thrombophilia. The most commonly inherited thrombophilia is factor V Leiden, which accounts for approximately 30% of cases of VTE caused by thrombophilia.2 Leiden refers to the city in the Netherlands where a variant of the normal clotting factor V was first described.3 Individuals with this genetic mutation are at increased risk for experiencing VTE as either deep venous thrombosis (DVT) or pulmonary embolism (PE). The treatment guidelines are not specific on the use of pharmacotherapy unless the patient has experienced an episode of VTE.
PATHOPHYSIOLOGY
Factor V is a procoagulant clotting factor that increases the production of thrombin and, by way of the clotting cascade, ultimately leads to clot formation. Factor V circulates in the plasma as an inactive factor and becomes activated when thrombin is introduced by proteolysis. Activated protein C is a natural anticoagulant that will degrade activated factor V (factor Va). This degradation reduces thrombin production and interrupts the clotting cascade. Factor V Leiden results from a single point mutation in the factor V gene that causes a poor anticoagulant response to activated protein C.
The inheritance of the defective gene may be expressed as either homozygous or heterozygous factor V Leiden. Heterozygosity carries a relative risk of incident VTE of approximately three-to-eight fold. In contrast, homozygosity carries a relative risk of incident VTE of 80-fold.4 Relative risk is used to calculate the odds that a thromboembolism will form. If the relative risk is greater than zero, it means that the odds favor a thromboembolism.5
DIAGNOSIS
The use of diagnostic testing is controversial because the etiology of thrombosis is multifactorial and the identification of a thrombophilic defect is only one of many elements that play a role in determining a patient’s risk.6 Factor V Leiden does not have distinct clinical features that will always be present. Instead, this diagnosis is generally suspected in individuals who have a history of VTE that manifested as either DVT or PE.2
Testing for thrombophilia is recommended when the results are needed to improve or modify management. The diagnosis of factor V Leiden requires completion of the activated protein C resistance test or a genetic test. Testing should be performed in the following patients: those who have VTE at any age under 50 years; those who have an unusual VTE location (hepatic, mesenteric, or cerebral veins), recurrent VTE, VTE plus a strong family history of thrombotic disease, VTE during pregnancy or during use of oral contraceptives; those who have a family history of VTE in relatives under age 50 years; and in female smokers under age 50 years who have had myocardial infarction.7
CURRENT TREATMENT GUIDELINES
Treatment of VTE should be individualized based on all of the risk factors that led to the clot and prevention of future clots. Current guidelines do not address empiric therapy with anticoagulants for those individuals who have not yet experienced thrombosis. The American College of Chest Physicians recommends anticoagulant therapy for VTE events that are provoked or unprovoked DVTs and PEs. The use of anticoagulant therapy for 3 months is recommended in the following instances: patients who experience proximal DVT of the leg or PE that is provoked by surgery; proximal DVT of the leg or PE provoked by a nonsurgical transient risk factor; patients who experience isolated distal DVT of the leg provoked by surgery or a nonsurgical transient risk factor; and in patients with an unprovoked DVT of the leg (isolated distal or proximal) or PE. In patients whose first VTE is unprovoked proximal DVT of the leg or PE and who have a low or moderate bleeding risk, the recommendation is extended anticoagulant therapy (no scheduled stop date); for those who have a high bleeding risk, the recommendation is 3 months of therapy.8
CASE REPORT
A 64-year-old white male presented with swelling and pain in his left leg and thigh. The patient denied any trauma to the leg or thigh but did notice some mild swelling earlier in the week. The patient was seen by his primary care provider and was found to have 2+ pitting edema throughout the leg and discomfort primarily in the calf and thigh. The patient had never had swelling like this before, and there was no swelling or pain in his right lower extremity. The patient had a history of peripheral vascular disease, but it presented as claudication (increased pain with walking). The patient denied having chest pain or shortness of breath, or any abdominal or urinary tract symptoms. The patient had no history of abnormal bleeding, bruising, or blood clots. He had not recently taken any long trips in a car or plane. A venous Doppler examination revealed subacute occlusive DVT in the left posterior tibial and gastrocnemius veins. There was also a subacute superficial thrombus in the communication between the great saphenous vein and the small saphenous vein.
Laboratory Results: Cardiolipin antibody immunoglobulin G was 4 (normal <15); anticardiolipin immunoglobulin M was 8 (normal <13), prothrombin factor II nucleotide was negative, factor V Leiden was positive (heterogeneous). Electrolytes, kidney function, and liver function tests were all normal, but fasting blood sugar was 108. A1C was 6.4%. Total cholesterol was 134 mg/dL; LDL cholesterol, 65 mg/dL; HDL cholesterol, 44 mg/dL; VLDL cholesterol, 25 mg/dL; cholesterol/HDL ratio, 3.0.
Past Medical History: Significant for coronary artery disease status post insertion of two stents in 2001, followed years later by two coronary bypass grafts in 2011; peripheral vascular disease status post stenting in the left femoral artery in 2007 and left iliac artery stent in 2015; left subclavian artery occlusion status post stent in 2012; right carotid vascular disease status post stent in 2007; history of hyperlipidemia, hypertension, and skin cancer (melanoma); left shoulder status postresection; history of chronic obstructive pulmonary disease, glucose intolerance, erectile dysfunction, and reflux esophagitis.
Other Surgeries: Spinal stenosis surgery L2-L3 in 2003; status post left knee replacement in 2004.
Social History: Married, employed as a delivery person for a local warehouse, history of smoking for 43 years, 1-2 packs per day, but quit 6 years ago. Nondrinker. History of polysubstance abuse.
Family History: History of strokes in mother at age 57 years and sister in her 50s; father died of a myocardial infarction in his 50s.
Current Medications: Metoprolol succinate XL 25 mg daily; atorvastatin 40 mg daily; lisinopril 10 mg daily; rivaroxaban 20 mg daily; nonprescription ranitidine 75 mg twice daily.
Discussion: Once the diagnosis of DVT in the left lower extremity was made, the patient was treated with rivaroxaban (Xarelto). He was told that since this was an unprovoked DVT and he was heterozygous for factor V Leiden, which is associated with activated protein C resistance, he was at significant risk for future venous thrombosis and would need lifetime anticoagulation therapy. The patient’s previous left knee replacement may have been a contributing factor to the formation of the clot.
PHARMACOTHERAPY
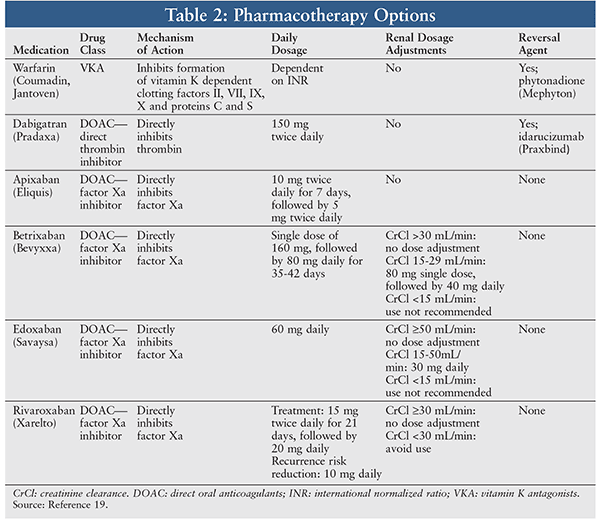
Current oral pharmacotherapy options available for coagulopathy due to factor V Leiden include vitamin K antagonists and direct oral anticoagulants (DOACs) such as direct thrombin inhibitors and factor Xa inhibitors. A summary of available agents in each class may be found in Table 2.
Vitamin K Antagonists
Warfarin: Warfarin is indicated for both the prophylaxis and treatment of venous thrombosis and PE.9 The use of warfarin will have no direct effect on an established thrombus, but the goal of anticoagulant therapy is to prevent further propagation of the clot and reduce the complication of the formed clot embolizing and traveling to another part of the body. Dosing of warfarin is individualized for each patient depending on the patient’s international normalized ratio (INR)—the standardized measure of time it takes for blood to clot—in response to the medication. Frequent monitoring of the INR is required until a patient is stabilized on a dose within the therapeutic INR range. Unfortunately, multiple foods, drugs, and other factors adversely affect this INR range. Warfarin is not recommended in pregnancy, as it may cause fetal harm. If a reversal agent is required, administration of phytonadione (Mephyton) at a dose of 2.5 to 25 mg is recommended.10
DOAC—Direct Thrombin Inhibitor
Dabigatran (Pradaxa): Dabigatran is a direct thrombin inhibitor that is indicated for the treatment of DVT and PE in patients who have been treated with a parenteral anticoagulant for 5 to 10 days.11 Additionally, it is indicated to reduce the risk of recurrence of DVT and PE in patients who have previously been treated. The recommended dosing in both instances is 150 mg orally twice daily. Dosage adjustments are not necessary for renal or hepatic impairment. A reversal agent such as idarucizumab (Praxbind) may be given at a dose of 5 g and administered as two doses of 2.5 g IV no more than 15 minutes apart.12
DOAC—Factor Xa Inhibitors
Use of this class of medications has grown because of the advantages of rapid onset, predictable pharmacokinetics, and limited food and drug interactions.13 In contrast, these agents have the disadvantages of high cost, limited availability of reversal agents, and inability to monitor their effects.
Apixaban (Eliquis): Apixaban is indicated for the treatment of DVT and PE and for reduction of the risk of recurrent DVT and PE following initial therapy. It does not require renal or hepatic dose adjustments for impairment. Use in pregnancy is not recommended because of the increased risk of hemorrhage in labor and delivery.14
Betrixaban (Bevyxxa): Betrixaban is indicated for the prophylaxis of VTE in adult patients who are at risk for thromboembolic complications while hospitalized for an acute medical illness that restricts mobility.15 The indication for this medication is specific, and the recommended treatment duration is 35 to 42 days. This medication is not recommended for extended-duration or lifelong therapy. Dosage adjustments are indicated if creatinine clearance is 15-30 mL/minute, and betrixaban is not recommended for use if creatinine clearance is <15 mL/minute. There is no dosage adjustment in hepatic impairment.
Edoxaban (Savaysa): Edoxaban is indicated for the treatment of DVT and PE following 5 to 10 days of therapy with a parenteral anticoagulant. Dosage adjustments may be made if creatinine clearance is between 15-30 mL/minute; if creatinine clearance is <15 mL/minute, use of this agent is not recommended. In moderate-to-severe hepatic impairment, use of this agent is not recommended.16
Rivaroxaban (Xarelto): Rivaroxaban is indicated for the treatment of DVT and PE plus reduction in risk of recurrence of DVT and/or PE in patients at risk for recurrent DVT and/or PE after completion of initial treatment lasting at least 6 months. Use in renal impairment (<30 mL/minute) is not recommended. Its use is also not recommended in cases of moderate-to-severe hepatic impairment.17
PHARMACIST’S ROLE
Pharmacists have received training that allows them to provide expert advice and recommendations for medication therapy. In the community setting, pharmacists are an accessible source of healthcare information for patients who inquire about factor V Leiden (See Patient Resources). Pharmacists are knowledgeable regarding the available anticoagulant options and are able to monitor for duplications of therapy or additional nonprescription agents that may put a patient at increased risk for bleeding complications. Properly educating patients on the risks and benefits surrounding each option is necessary in order for them to make informed decisions regarding their care.
REFERENCES
1. CDC. Venous thromboembolism (blood clots). Updated June 22, 2015. www.cdc.gov/ncbddd/dvt/data.html. Accessed January 30, 2018.
2. Kujovich JL. Factor V Leiden thrombophilia. Updated January 4, 2018. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews® [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2018.
3. National Blood Clot Alliance. What is factor V Leiden? www.stoptheclot.org/risk_factors/what_is_factor_v_leiden.htm. Accessed January 30, 2018.
4. Folsom AR, Cushman M, Tsai MY, et al. A prospective study of venous thromboembolism in relation to factor V Leiden and related factors. Blood. 2002;99(8):2720-2725.
5. Irwig L, Irwig J, Trevena L, et al. Chapter 18. Relative risk, relative and absolute risk reduction, number needed to treat and confidence intervals.
Smart Health Choices: Making Sense of Health Advice. London, England. Hammersmith Press; 2008.
6. Stevens SM, Woller SC, Bauer KA, et al. Guidance for the evaluation and treatment of hereditary and acquired thrombophilia. J Thromb Thrombolysis. 2016;41(1):154-164.
7. Grody WW, Griffin JH, Taylor AK, et al. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med. 2001;3(2):139-148.
8. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
9. Coumadin (warfarin) package insert. Princeton, NJ: Bristol-Myers Squibb Co; August 2017.
10. Mephyton (phytonadione) package insert. Whitehouse Station, NJ: Merck & Co, Inc; April 2004.
11. Pradaxa (dabigatran) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; July 2017.
12. Praxbind (idarucizumab) package insert. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; October 2015.
13. Cook RM, Rondina MT, Horton DJ. Ivaroxaban for the long-term treatment of spontaneous ovarian vein thrombosis caused by factor V Leiden homozygosity. Ann Pharmacother. 2014;48(8):1055-1060.
14. Eliquis (apixaban) package insert. Princeton, NJ: Bristol-Myers Squibb Co; July 2016.
15. Bevyxxa (betrixaban) package insert. South San Francisco, CA: Portola Pharmaceuticals, Inc;
June 2017.
16. Edoxaban (Savaysa) package insert. Tokyo, Japan: Daiichi Sankyo Co, LTD; January 2015.
17. Rivaroxaban (Xarelto) package insert. Titusville, NJ: Janssen Pharmaceuticals Inc; October 2017.
18. Ornstein DL, Cushman M. Cardiology patient page. Factor V Leiden. Circulation. 2003;107(15):e94-e97.
19. Lexicomp Online. Hudson, Ohio: Lexi-Comp, Inc.; 2013. http://online.lexi.com. Accessed December 18, 2017.
To comment on this article contact rdavidson@uspharmacist.com.